
Exhibit 99.1

Ticker Symbol OTCQB:RLMD Innovations in Central Nervous System Diseases April 4, 2018 Targeting Major Advances in Treatment of CNS Disorders Ticker Symbol OTCQB:RLMD

Forward Looking Statements Certain statements contained in this presentation or in other documents of Relmada Therapeutics (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward - looking statements . ” These statements can be identified by the fact that they do not relate strictly to historic or current facts . They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition . These statements are based upon the current beliefs and expectations of the Company’s management and are subject to significant risks and uncertainties . Statements regarding future action, future performance and/or future results including, without limitation, those relating to the timing for completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formulations and products and regulatory filings related to the same may differ from those set forth in the forward - looking statements . Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate . Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward - looking statement . It is not possible to predict or identify all such risks, contingencies and uncertainties . The Company identifies some of these factors in its Securities and Exchange Commission (“SEC”) filings on Forms 10 - K, 10 - Q and 8 - K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties effecting the Company and its business and financial performance . The Company assumes no obligation to update forward - looking statements as circumstances change . Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10 - K, 10 - Q and 8 - K reports . 2

• Relmada is a global leader in advancing dextromethadone (REL - 1017) as a rapid - acting oral treatment for depression, CNS and ophthalmological disorders. • Demonstrated NMDA receptor binding activity in preclinical studies • Mechanism of Action ( MoA ) shows potential to repair damaged brain cell (neuron) connections and build new connections through modulation of the NMDA receptor pathway, the underlying basis of several CNS diseases. • Clinical results thus far indicate a highly favorable safety and tolerability profile. • Strong efficacy signal in depression established in three independent animal models. • Phase 2 depression trial under way; data expected H1 2019. Unlocking the Potential of Dextromethadone 3
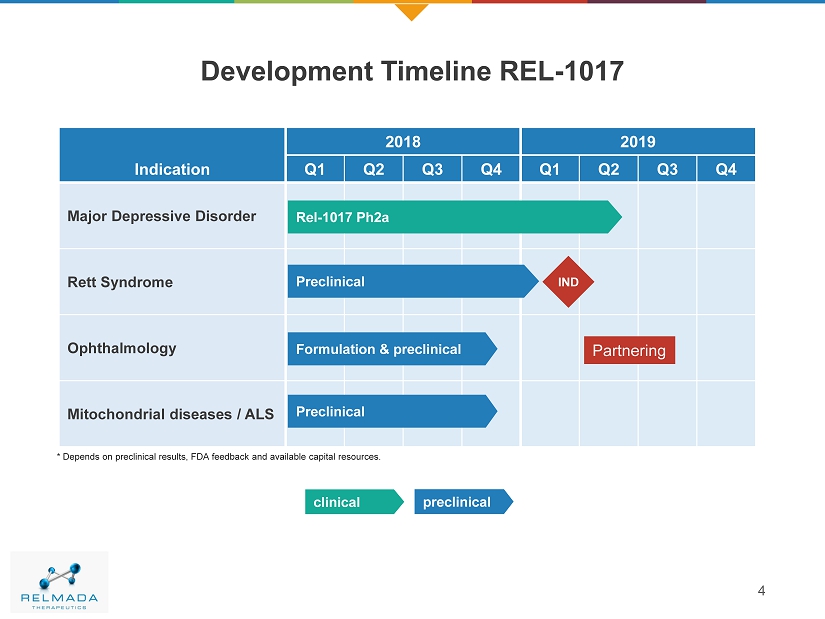
4 Development Timeline REL - 1017 Indication 2018 2019 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Majo r Depressive Disorder Rett Syndrome Ophthalmology Mitochondrial diseases / ALS Rel - 1017 Ph2a Preclinical preclinical clinical Preclinical Formulation & preclinical * Depends on preclinical results, FDA feedback and available capital resources. IND Partnering

Ticker Symbol OTCQB:RLMD Dextromethadone (REL - 1017) as a Treatment for Major Depressive Disorder

Expanding Focus on NMDA Role in Treatment of Depression 6 6 Johnson & Johnson Is Reinventing the Party Drug Ketamine to Treat Depression
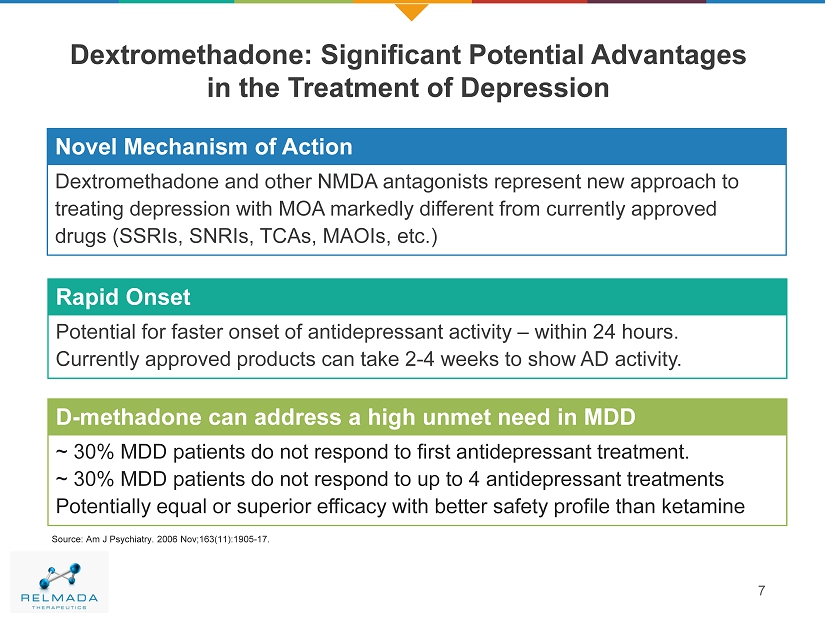
Dextromethadone: Significant Potential Advantages in the Treatment of Depression 7 Source : Am J Psychiatry. 2006 Nov;163(11):1905 - 17. Novel MOA Novel Mechanism of Action Dextromethadone and other NMDA antagonists represent new approach to treating depression with MOA markedly different from currently approved drugs (SSRIs, SNRIs, TCAs, MAOIs, etc.) Rapid Onset Potential for faster onset of antidepressant activity – within 24 hours. Currently approved products can take 2 - 4 weeks to show AD activity. D - methadone can address a high unmet need in MDD ~ 30% MDD patients do not respond to first antidepressant treatment. ~ 30% MDD patients do not respond to up to 4 antidepressant treatments Potentially equal or superior efficacy with better safety profile than ketamine
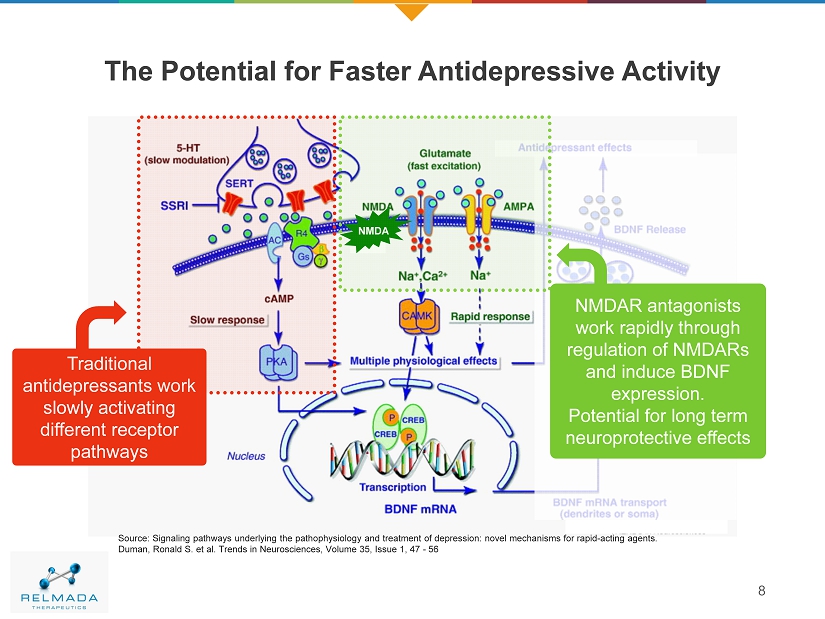
The Potential for Faster Antidepressive Activity 8 Source: Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid - acting agents. Duman, Ronald S. et al. Trends in Neurosciences, Volume 35, Issue 1, 47 - 56 NMDA Traditional antidepressants work slowly activating different receptor pathways NMDAR antagonists work rapidly through regulation of NMDARs and induce BDNF expression. Potential for long term neuroprotective effects
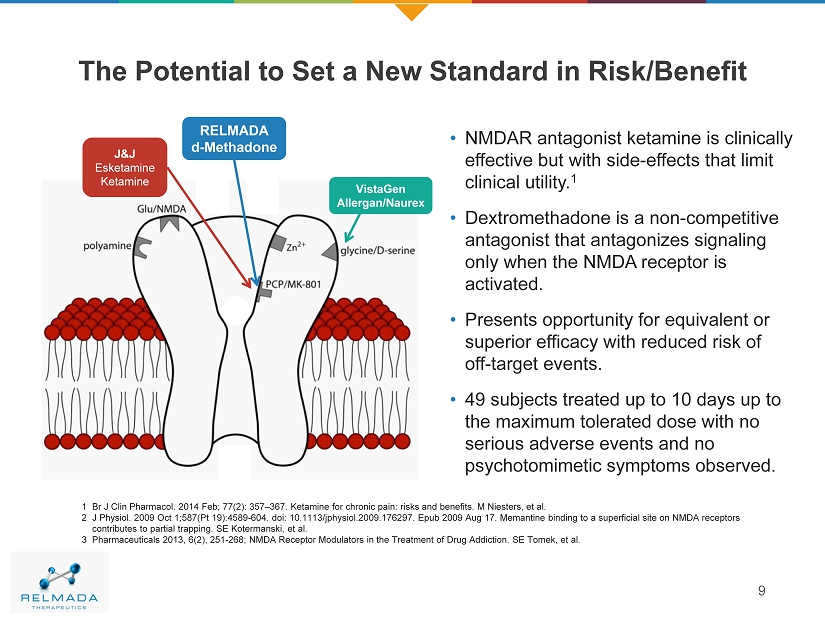
The Potential to Set a New Standard in Risk/Benefit • NMDAR antagonist ketamine is clinically effective but with side - effects that limit clinical utility. 1 • Dextromethadone is a non - competitive antagonist that antagonizes signaling only when the NMDA receptor is activated. • Presents opportunity for equivalent or superior efficacy with reduced risk of off - target events. • 49 subjects treated up to 10 days up to the maximum tolerated dose with no serious adverse events and no psychotomimetic symptoms observed . 1 Br J Clin Pharmacol. 2014 Feb; 77(2): 357 – 367. Ketamine for chronic pain: risks and benefits. M Niesters, et al. 2 J Physiol. 2009 Oct 1;587(Pt 19):4589 - 604. doi: 10.1113/jphysiol.2009.176297. Epub 2009 Aug 17. Memantine binding to a superfi cial site on NMDA receptors contributes to partial trapping. SE Kotermanski, et al. 3 Pharmaceuticals 2013, 6(2), 251 - 268; NMDA Receptor Modulators in the Treatment of Drug Addiction. SE Tomek, et al. VistaGen Allergan/Naurex RELMADA d - Methadone J&J Esketamine Ketamine 9
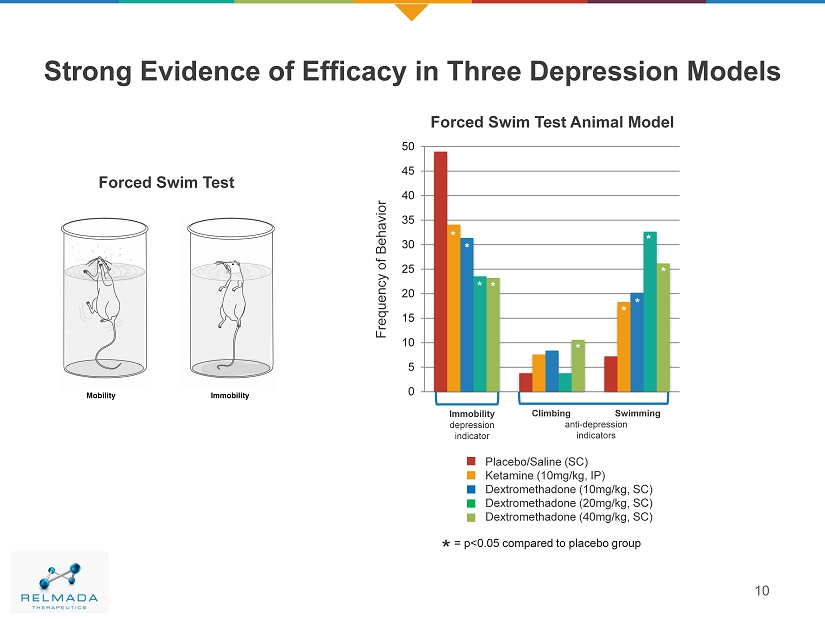
0 5 10 15 20 25 30 35 40 45 50 Strong Evidence of Efficacy in Three Depression Models 10 Frequency of Behavior Immobility depression indicator Climbing Swimming anti - depression indicators Placebo/Saline (SC) Ketamine (10mg/kg, IP) Dextromethadone (10mg/kg, SC) Dextromethadone (20mg/kg, SC) Dextromethadone (40mg/kg, SC) * Forced Swim Test Animal Model Forced Swim Test * * * * * * * * = p<0.05 compared to placebo group *
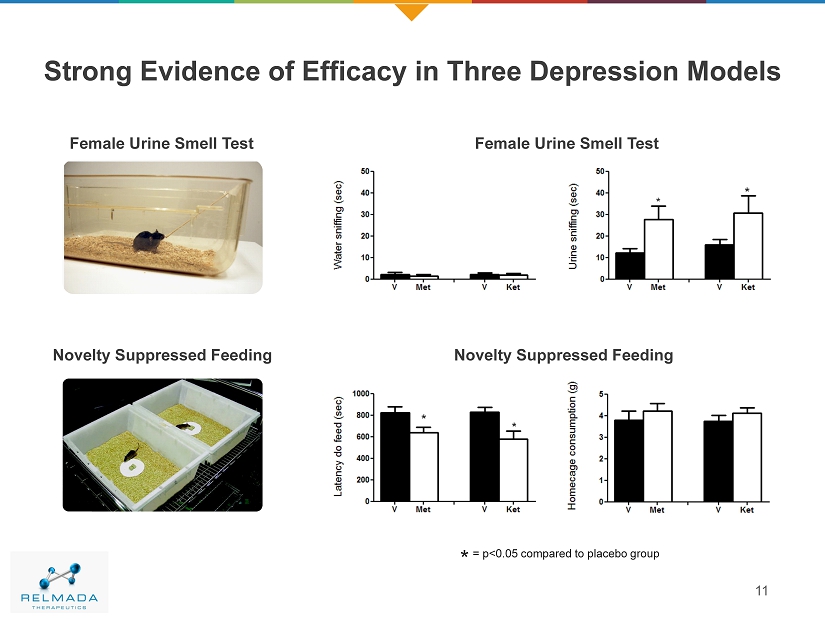
Novelty Suppressed Feeding * * Strong Evidence of Efficacy in Three Depression Models 11 * Female Urine Smell Test * Female Urine Smell Test Novelty Suppressed Feeding = p<0.05 compared to placebo group *
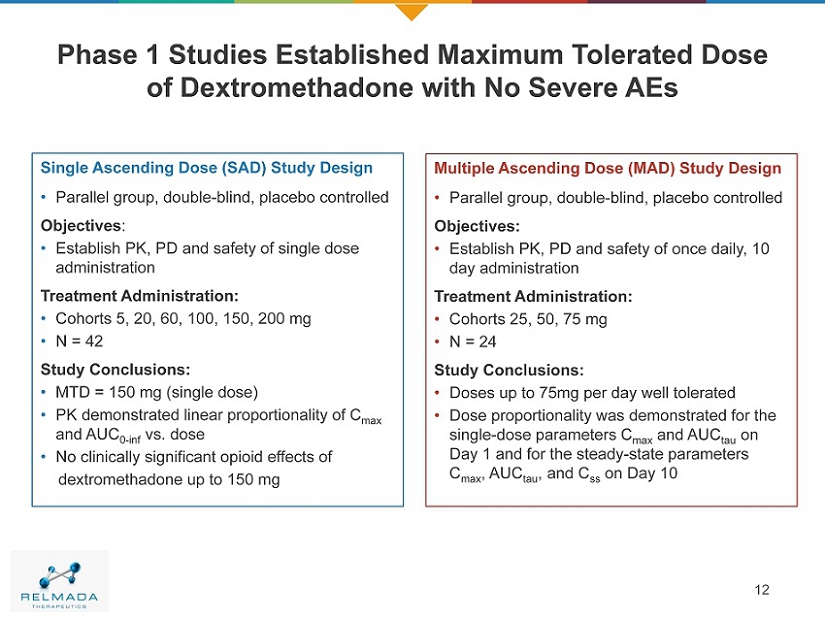
Phase 1 Studies Established Maximum Tolerated Dose of Dextromethadone with No Severe AEs 12 Single Ascending Dose (SAD) Study Design • Parallel group, double - blind, placebo controlled Objectives : • Establish PK, PD and safety of single dose administration Treatment Administration: • Cohorts 5, 20, 60, 100, 150, 200 mg • N = 42 Study Conclusions: • MTD = 150 mg (single dose) • PK demonstrated linear proportionality of C max and AUC 0 - inf vs. dose • No clinically significant opioid effects of dextromethadone up to 150 mg Multiple Ascending Dose (MAD) Study Design • Parallel group, double - blind, placebo controlled Objectives: • Establish PK, PD and safety of once daily, 10 day administration Treatment Administration: • Cohorts 25, 50, 75 mg • N = 24 Study Conclusions: • Doses up to 75mg per day well tolerated • Dose proportionality was demonstrated for the single - dose parameters C max and AUC tau on Day 1 and for the steady - state parameters C max , AUC tau , and C ss on Day 10 12
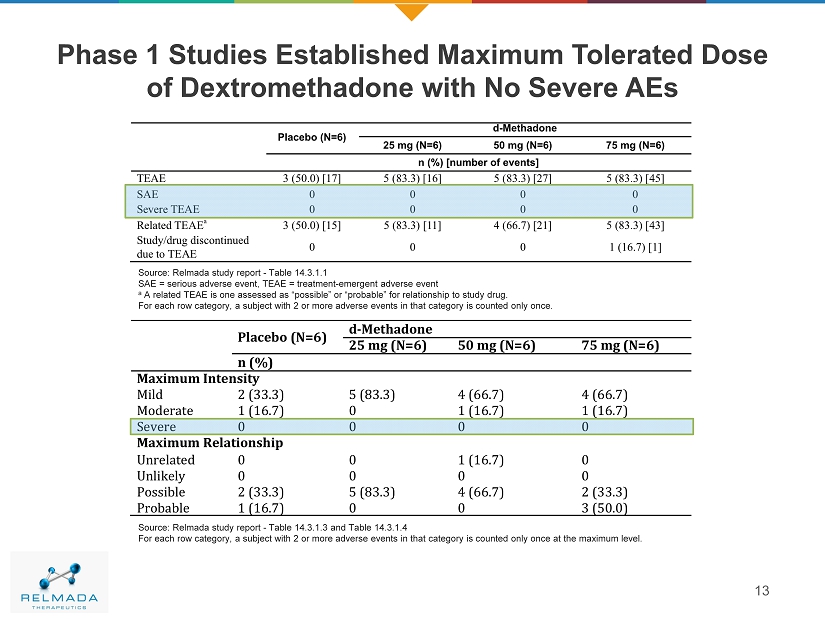
Phase 1 Studies Established Maximum Tolerated Dose of Dextromethadone with No Severe AEs 13 Source: Relmada study report - Table 14.3.1.1 SAE = serious adverse event, TEAE = treatment - emergent adverse event a A related TEAE is one assessed as “possible” or “probable” for relationship to study drug. For each row category, a subject with 2 or more adverse events in that category is counted only once. Source: Relmada study report - Table 14.3.1.3 and Table 14.3.1.4 For each row category, a subject with 2 or more adverse events in that category is counted only once at the maximum level.
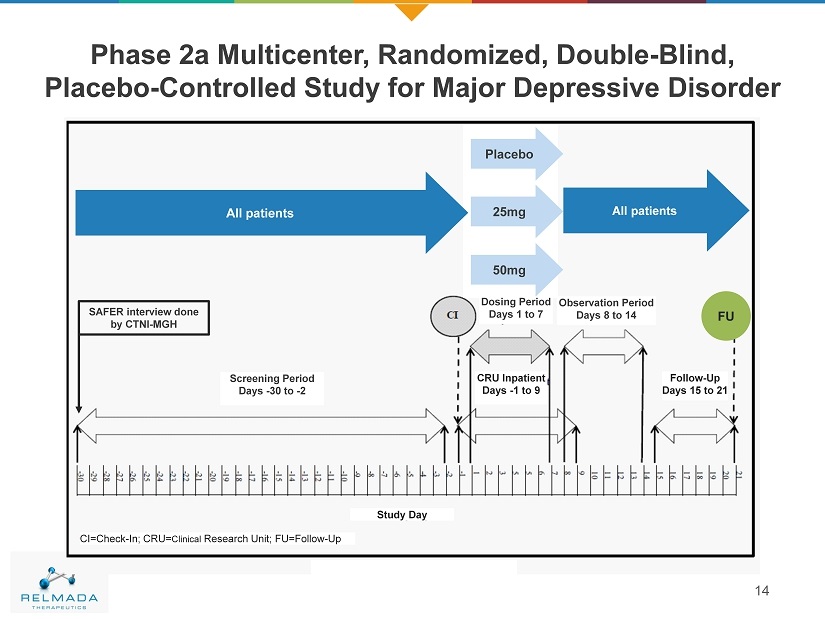
Phase 2a Multicenter, Randomized, Double - Blind, Placebo - Controlled Study for Major Depressive Disorder 14 SAFER interview done by CTNI - MGH Screening Period Days - 30 to - 2 Dosing Period Days 1 to 7 Observation Period Days 8 to 14 Follow - Up Days 15 to 21 CRU Inpatient Days - 1 to 9 All Patients All Patients Study Day CI=Check - In; CRU= Clinical Research Unit; FU=Follow - Up FU Placebo 25mg 50mg All patients All patients
• Treatment in non - responding MDD patients. • Randomized, double - blind, placebo - controlled study of 7 - day dosing at 25 mg and 50 mg QD as adjunctive therapy. • Dose selection based on effect measured in pre - clinical studies. • ~60 eligible subjects randomized to three arms: placebo, 25 mg, 50 mg. • Primary Endpoints – Assess the safety and tolerability vs. placebo as adjunctive treatment in patients with MDD non responding to antidepressants • Secondary Endpoints – Efficacy as adjunctive treatment in patients with MDD non - responding to antidepressants – Characterize the pharmacokinetic profile of 25 mg and 50 mg of REL - 1017 (dextromethadone) as adjunctive treatment in patients with MDD non - responding to antidepressants REL - 1017 - 202 – Phase 2 Study 15

• 20 subjects/arm designed to show clinically relevant difference between dextromethadone and placebo • Efficacy endpoints cover all major depression symptoms and will provide information on totality of antidepressant effect • Study will inform the design of pivotal studies REL - 1017 - 202 Trial Protocol Advantages 16
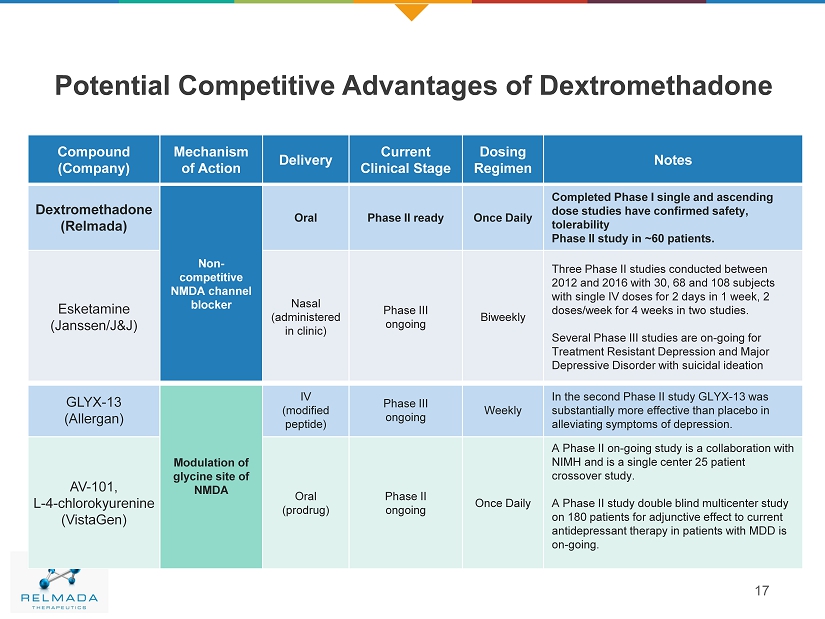
Potential Competitive Advantages of Dextromethadone 17 Compound (Company) Mechanism of Action Delivery Current Clinical Stage Dosing Regimen Notes Dextromethadone (Relmada) Non - competitive NMDA channel blocker Oral Phase II ready Once Daily Completed Phase I single and ascending dose studies have confirmed safety, tolerability Phase II study in ~60 patients. Esketamine (Janssen/J&J) Nasal (administered in clinic) Phase III ongoing Biweekly Three Phase II studies conducted between 2012 and 2016 with 30, 68 and 108 subjects with single IV doses for 2 days in 1 week, 2 doses/week for 4 weeks in two studies . Several Phase III studies are on - going for Treatment Resistant Depression and Major Depressive Disorder with suicidal ideation GLYX - 13 (Allergan) Modulation of glycine site of NMDA IV (modified peptide) Phase III ongoing Weekly In the second Phase II study GLYX - 13 was substantially more effective than placebo in alleviating symptoms of depression. AV - 101, L - 4 - chlorokyurenine ( VistaGen ) Oral (prodrug) Phase II ongoing Once Daily A Phase II on - going study is a collaboration with NIMH and is a single center 25 patient crossover study . A Phase II study double blind multicenter study on 180 patients for adjunctive effect to current antidepressant therapy in patients with MDD is on - going.
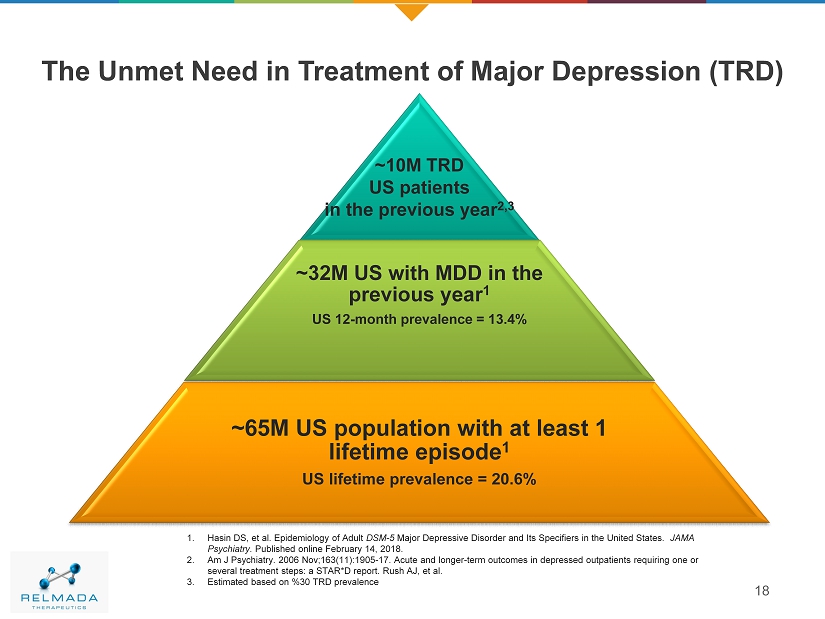
The Unmet Need in Treatment of Major Depression (TRD) 18 ~10M TRD US patients in the previous year 2,3 ~32M US with MDD in the previous year 1 US 12 - month prevalence = 13.4% ~65M US population with at least 1 lifetime episode 1 US lifetime prevalence = 20.6% 1. Hasin DS, et al. Epidemiology of Adult DSM - 5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry. Published online February 14, 2018. 2. Am J Psychiatry. 2006 Nov;163(11):1905 - 17. Acute and longer - term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Rush AJ, et al . 3. Estimated based on %30 TRD prevalence

Ticker Symbol OTCQB:RLMD Dextromethadone (REL - 1017) as a Treatment for Rett Syndrome

• X - linked neurodevelopmental disorder with high unmet need • Caused by Mecp2 gene mutation – Loss of Mecp2 disrupts synaptic function & structure and neuronal networks – Short period of developmental stagnation, then rapid regression in language and motor skills, followed by long - term stability. • Orphan Disease – Affecting ~15,000 in U.S., primarily girls – Established network of specialized Rett clinics, patients registry, and strong advocacy community to support clinical research • No approved therapy – Not amenable to gene or protein replacement therapy approaches Rett Syndrome 20

• Studies of ketamine in Rett Syndrome mouse models show that: – Low - dose ketamine acutely reverses multiple disease manifestations – Chronic administration of ketamine improves Rett Syndrome progression • Mecp2 mouse models recapitulate salient clinical manifestations allowing for translatability of pre - clinical findings. • Dr. Michela Fagiolini at Harvard Medical School and Boston Children’s Hospital conducting acute and chronic studies in Mecp2 +/ - female mice. • Relmada toxicology studies in juvenile animals proceeding in parallel. • Fast development path – Potential for entry directly into Phase 2 in 2019 NMDARs Have Shown Potential Efficacy in Treatment of Rett Syndrome 21
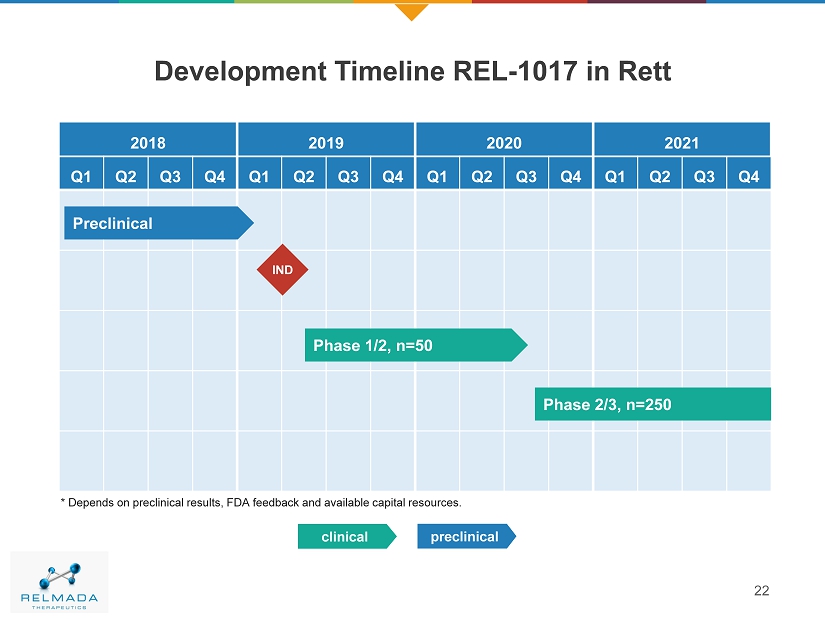
22 Development Timeline REL - 1017 in Rett 2018 2019 2020 2021 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Phase 1/2, n=50 Preclinical * Depends on preclinical results, FDA feedback and available capital resources. Phase 2/3, n=250 preclinical clinical IND

Ticker Symbol OTCQB:RLMD Dextromethadone (REL - 1017): Targeting Additional Potential Indications
• Ophthalmology – Formulation work underway – Animal studies planned H2 2018 – Potential for multiple indications – Partnering candidate • Mitochondrial – Animal studies underway • Amyotrophic lateral sclerosis (ALS) MOA Shows Potential in Ophthalmologic, Mitochondrial Indications and ALS 24

Ticker Symbol OTCQB:RLMD Additional Relmada Assets
• Extended release, abuse deterrent, proprietary formulation of the opioid analgesic levorphanol – strong opioid with greater potency than morphine. – demonstrated broad spectrum of analgesic activity against many different types of pain including neuropathic pain, post - surgical pain, and chronic pain in patients refractory to other opioids – binds to all three opioid receptor subtypes involved in analgesia, the NMDA receptor and the norepinephrine and serotonin uptake pumps • REL - 1015 is: – Abuse deterrent – Clinically and pharmacologically differentiated from other strong opioids – Effective in nociceptive and neuropathic pain – 505(b)(2) regulatory pathway – Potential for once or twice daily extended release formulation • Completed Phase 1 REL - 1015 Levocap ER 26
• Novel oral formulation of modified release buprenorphine for chronic pain and opioid dependence indications – Overcomes buprenorphine first pass metabolism in the upper gastrointestinal tract to allow for oral administration in traditional capsule or tablet form • REL - 1028 is – Potentially the first traditional tablet form of buprenorphine designed to deliver safe and effective blood levels – Potential uses: addiction and management of moderate to severe chronic pain – Schedule - III opioid; reduced risk of abuse and physical dependence – 505(b)(2) regulatory pathway – Can go directly into Phase III (no Phase II study required) • Completed singe dose PK study, 18 patients REL - 1028 BuTab 27

• Novel version of local anesthetic mepivacaine for treatment of painful peripheral neuropathies – Potential treatment for diabetic neuropathy, postherpetic neuralgia and HIV - associated neuropathy • Blocks the nerve impulses that send pain signals to the brain • REL - 1021: – Has received FDA orphan drug designations in postherpetic neuralgia and HIV - associated neuropathy – 505(b)(2) regulatory pathway – Potential for mono or combination therapy – Gel formulation provides a number of advantages over existing patch administration technology • Completed preclinical toxicology studies REL - 1021 MepiGel 28

Ticker Symbol OTCQB:RLMD Corporate Information
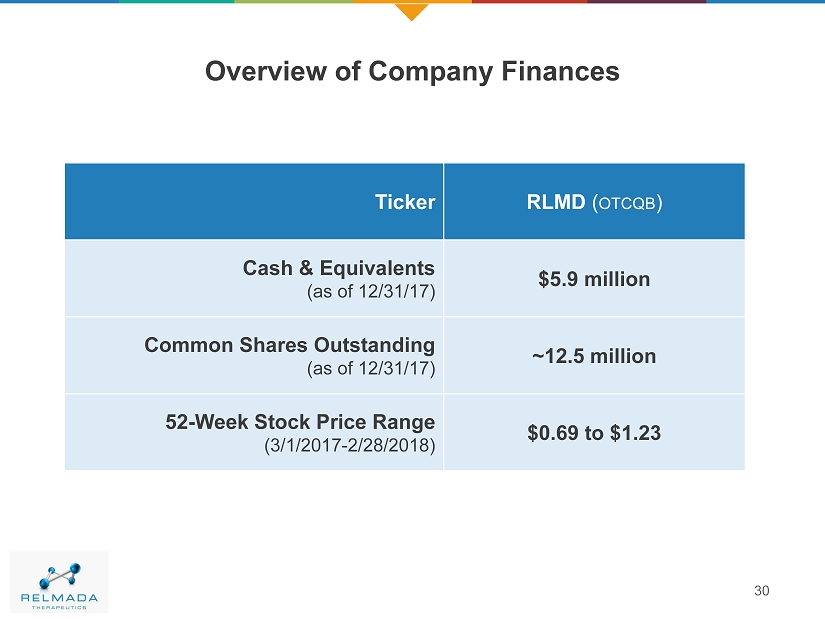
Overview of Company Finances Ticker RLMD ( OTCQB ) Cash & Equivalents (as of 12/31/17) $5.9 million Common Shares Outstanding (as of 12/31/17) ~12.5 million 52 - Week Stock Price Range (3/1/2017 - 2/28/2018) $0.69 to $1.23 30

• Strong clinical evidence supporting continued development of dextromethadone (REL - 1017) in multiple indications. • Clinical programs with clear regulatory pathways. • Established network of clinical researchers to support development program. • Strong IP with protection to the mid - 2030s. • Targeting multiple areas of unmet need that represent significant commercial opportunities. • Multiple major pending milestones in the next 12 - 18 months, including P2a data in H1 2019, Rett syndrome pre - clinical proof of concept, and Nasdaq listing. Relmada: Positioned for Success 31

750 Third Avenue, 9 th Floor New York, NY 10017 www.relmada.com Email: info@relmada.com Targeting Major Advances in Treatment of CNS Disorders Ticker Symbol OTCQB:RLMD