
Exhibit 99.1

Targeting Major Advances in Treatment of CNS Disorders November 23, 2021 I Nasdaq: RLMD 1

Disclosures Certain statements contained in this presentation or in other documents of Relmada Therapeutics, Inc. (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward - lookin g statements.” These statements can be identified by the fact that they do not relate strictly to historic or current facts. Th ey use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar mea nin g in connection with any discussion of future operating or financial performance or condition. These statements are based upon the cu rrent beliefs and expectations of the Company’s management and are subject to significant risks and uncertainties. Statements regar din g future action, future performance and/or future results, those relating to the timing for completion, and results of, schedul ed or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results ( if any) of the Company’s formulations and products and regulatory filings related to the same may differ from those set forth in the for war d - looking statements. Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate. Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward - looking statement. It is not possible to predict or identify all such risks, contingencies and uncertainties. The Company identifies some of these factors in its S ecu rities and Exchange Commission (“SEC”) filings on Forms 10 - K, 10 - Q and 8 - K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties affecting the Company and its business and finan cia l performance. The Company assumes no obligation to update forward - looking statements as circumstances change. Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10 - K, 10 - Q and 8 - K reports. 2

Investment Highlights Highly Compelling Opportunity in REL - 1017 Multiple Catalysts Expected Over Next 18 Months CNS Focus with Lead Program in Major Depressive Disorder (MDD) CNS= Central Nervous System **Our fiscal year end is December 31. The periods referred to in this slide are calendar years and quarters. 3
Major Depressive Disorder & REL - 1017
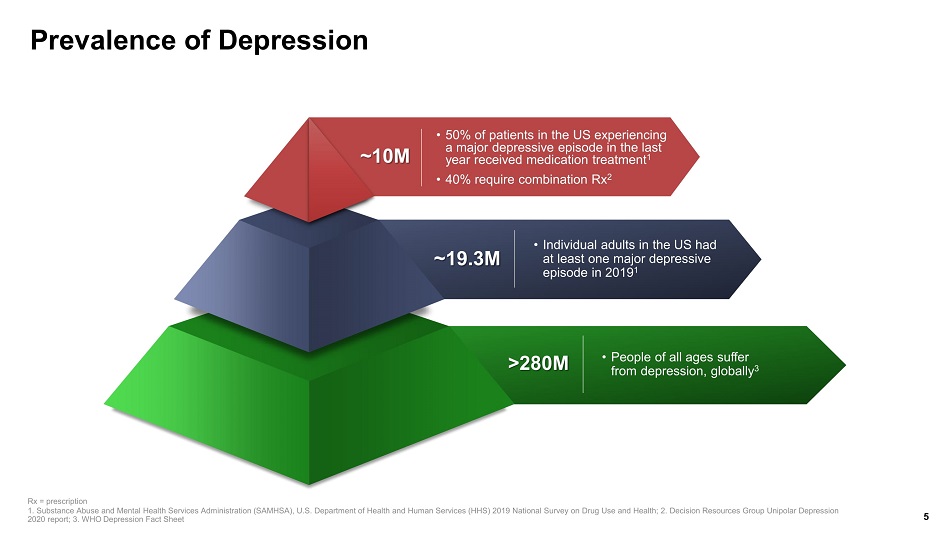
Prevalence of Depression ~10M • 50% of patients in the US experiencing a major depressive episode in the last year received medication treatment 1 • 40% require combination Rx 2 ~19.3M • Individual adults in the US had at least one major depressive episode in 2019 1 >280M • People of all ages suffer from depression, globally 3 5 Rx = prescription 1. Substance Abuse and Mental Health Services Administration (SAMHSA), U.S. Department of Health and Human Services (HHS) 201 9 N ational Survey on Drug Use and Health; 2. Decision Resources Group Unipolar Depression 2020 report; 3. WHO Depression Fact Sheet
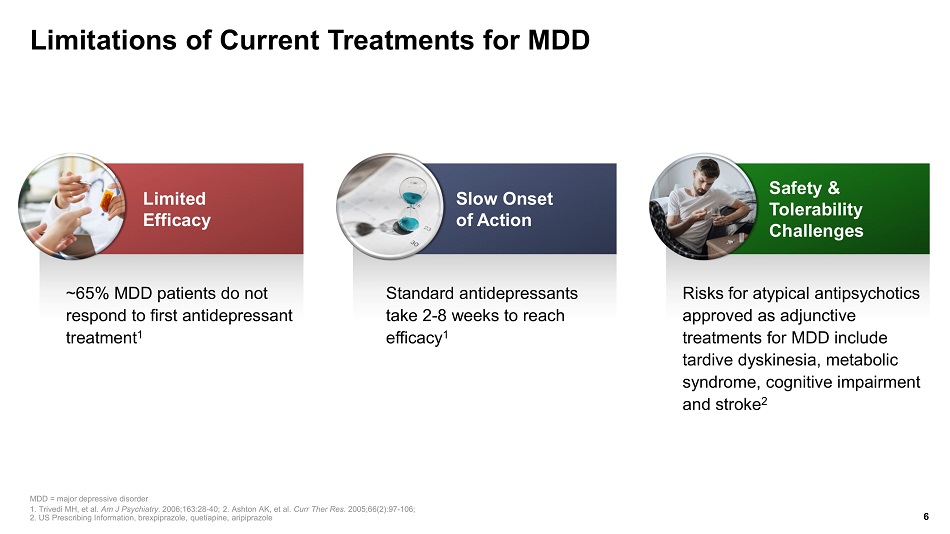
Limitations of Current Treatments for MDD ~65% MDD patients do not respond to first antidepressant treatment 1 Standard antidepressants take 2 - 8 weeks to reach efficacy 1 MDD = major depressive disorder 1. Trivedi MH, et al. Am J Psychiatry. 2006;163:28 - 40; 2. Ashton AK, et al. Curr Ther Res. 2005;66(2):97 - 106; 2. US Prescribing Information, brexpiprazole , quetiapine, aripiprazole 6 Slow Onset of Action Risks for atypical antipsychotics approved as adjunctive treatments for MDD include tardive dyskinesia, metabolic syndrome, cognitive impairment and stroke 2 Safety & Tolerability Challenges Limited Efficacy
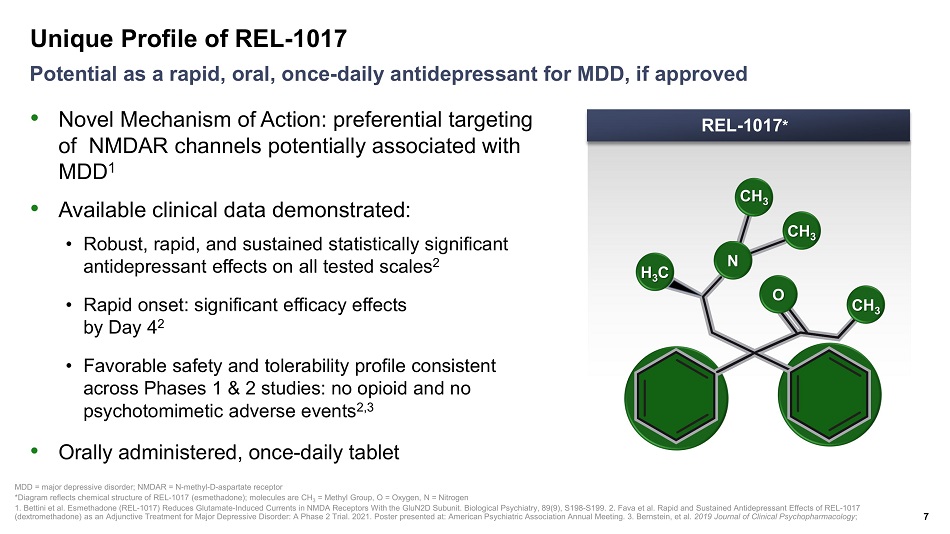
Unique Profile of REL - 1017 7 Potential as a rapid, oral, once - daily antidepressant for MDD, if approved MDD = major depressive disorder; NMDAR = N - methyl - D - aspartate receptor *Diagram reflects chemical structure of REL - 1017 ( esmethadone ); molecules are CH 3 = Methyl Group, O = Oxygen, N = Nitrogen 1. Bettini et al. Esmethadone (REL - 1017) Reduces Glutamate - Induced Currents in NMDA Receptors With the GluN2D Subunit. Biologica l Psychiatry, 89(9), S198 - S199. 2. Fava et al. Rapid and Sustained Antidepressant Effects of REL - 1017 ( dextromethadone ) as an Adjunctive Treatment for Major Depressive Disorder: A Phase 2 Trial. 2021. Poster presented at: American Psychiatric Ass ociation Annual Meeting. 3. Bernstein, et al. 2019 Journal of Clinical Psychopharmacology ; REL - 1017 * CH 3 CH 3 CH 3 O N H 3 C • Novel Mechanism of Action: preferential targeting of NMDAR channels potentially associated with MDD 1 • Available clinical data demonstrated: • Robust, rapid, and sustained statistically significant antidepressant effects on all tested scales 2 • Rapid onset: significant efficacy effects by Day 4 2 • Favorable safety and tolerability profile consistent across Phases 1 & 2 studies: no opioid and no psychotomimetic adverse events 2,3 • Orally administered, once - daily tablet

REL - 1017 Ph 1 & 2 Efficacy & Safety Data
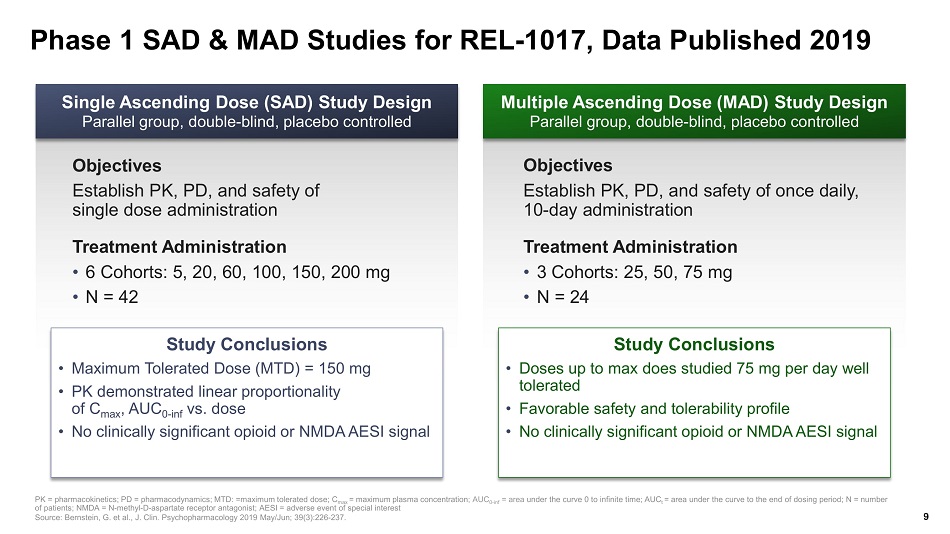
Phase 1 SAD & MAD Studies for REL - 1017, Data Published 2019 PK = pharmacokinetics; PD = pharmacodynamics; MTD: =maximum tolerated dose; C max = maximum plasma concentration ; AUC 0 - inf = area under the curve 0 to infinite time; AUC t = area under the curve to the end of dosing period; N = number of patients; NMDA = N - methyl - D - aspartate receptor antagonist; AESI = adverse event of special interest Source: Bernstein, G. et al., J. Clin. Psychopharmacology 2019 May/Jun; 39(3):226 - 237. Multiple Ascending Dose (MAD) Study Design Parallel group, double - blind, placebo controlled Single Ascending Dose (SAD) Study Design Parallel group, double - blind, placebo controlled Objectives Establish PK, PD, and safety of single dose administration Treatment Administration • 6 Cohorts: 5, 20, 60, 100, 150, 200 mg • N = 42 Objectives Establish PK, PD, and safety of once daily, 10 - day administration Treatment Administration • 3 Cohorts: 25, 50, 75 mg • N = 24 Study Conclusions • Maximum Tolerated Dose (MTD) = 150 mg • PK demonstrated linear proportionality of C max , AUC 0 - inf vs. dose • No clinically significant opioid or NMDA AESI signal Study Conclusions • Doses up to max does studied 75 mg per day well tolerated • Favorable safety and tolerability profile • No clinically significant opioid or NMDA AESI signal 9
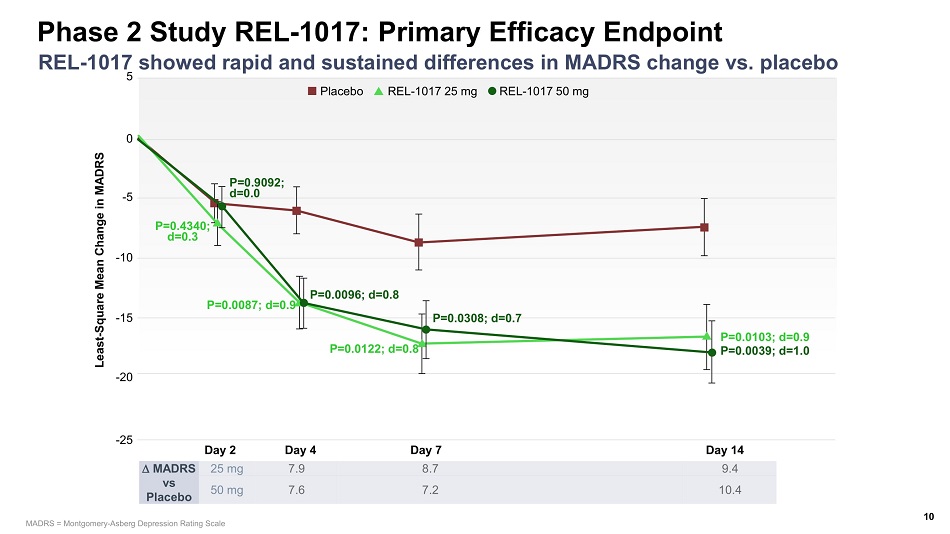
Day 2 Day 4 Day 7 Day 14 5 0 - 5 - 25 Least - Square Mean Change in MADRS - 10 - 15 - 20 P=0.0103; d=0.9 P=0.0039; d=1.0 P=0.0308; d=0.7 P=0.0122; d=0.8 P=0.0096; d=0.8 P=0.0087; d=0.9 P=0.9092; d=0.0 P=0.4340; d=0.3 Placebo REL - 1017 25 mg REL - 1017 50 mg Phase 2 Study REL - 1017: Primary Efficacy Endpoint 10 REL - 1017 showed rapid and sustained differences in MADRS change vs. placebo MADRS vs Placebo 25 mg 7.9 8.7 9.4 50 mg 7.6 7.2 10.4 MADRS = Montgomery - Asberg Depression Rating Scale

Safety & Tolerability Findings from Phase 2 • Only Mild and Moderate AEs – no SAEs • No increased prevalence of specifically relevant organ group AEs in treatment groups vs placebo • No evidence of opiate effects or withdrawal symptoms in treatment groups vs placebo • No evidence of treatment - induced dissociative or psychotomimetic symptoms in the treatment groups vs placebo 11 Safety & Tolerability Comparable to Placebo AE = adverse event; SAE = serious adverse event Source: Fava et al. Rapid and Sustained Antidepressant Effects of REL - 1017 ( dextromethadone ) as an Adjunctive Treatment for Major Depressive Disorder: A Phase 2 Trial. 2021. Poster presented at: American Psychiatric Ass ociation Annual Meeting

Human Abuse Potential (HAP) Study of REL - 1017 vs. Oxycodone

Human Abuse Potential (HAP) Study of REL - 1017 13 HAP Studies, per FDA’s Guidance for Industry 1 : The HAP Program for REL - 1017 includes two studies: 1 Assessment of Abuse Potential of Drugs Guidance for Industry 2017 https:// www.fda.gov /media/116739/download • Typically required for CNS - active drugs • Should be conducted in experienced recreational drug users • Use standardized questionnaires at specific timepoints • Positive controls should be FDA - approved controlled substances, that are pharmacologically similar to the test drug • This assessment is included in the New Drug Application (NDA) and used in determination of drug scheduling • Mu Opioid Agonist HAP • Oxycodone as active control • Completed in July 2021 • NMDA Receptor Antagonist HAP • Ketamine as active control • Top - line data expected in 1Q 22
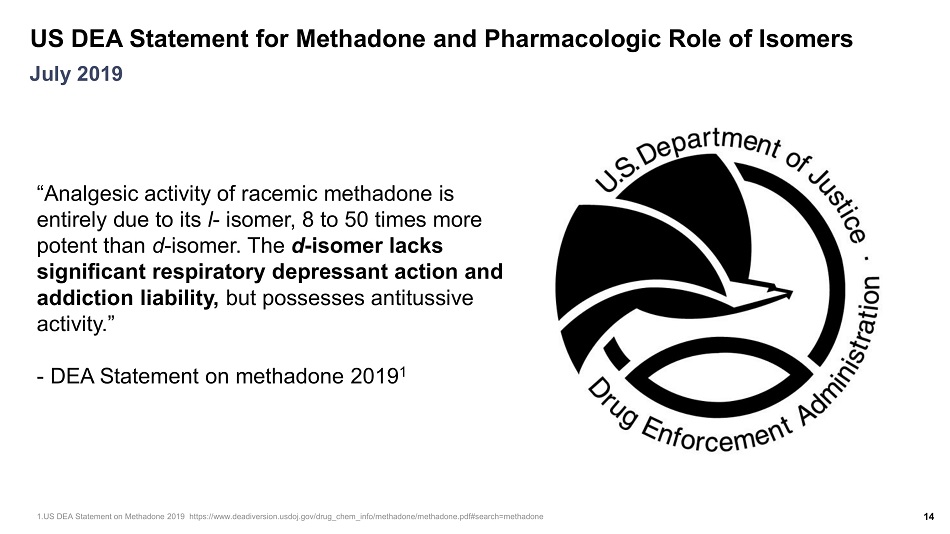
US DEA Statement for Methadone and Pharmacologic Role of Isomers 14 July 2019 “Analgesic activity of racemic methadone is entirely due to its l - isomer, 8 to 50 times more potent than d - isomer. The d - isomer lacks significant respiratory depressant action and addiction liability, but possesses antitussive activity.” - DEA Statement on methadone 2019 1 1.US DEA Statement on Methadone 2019 https:// www.deadiversion.usdoj.gov / drug_chem_info /methadone/ methadone.pdf#search =methadone
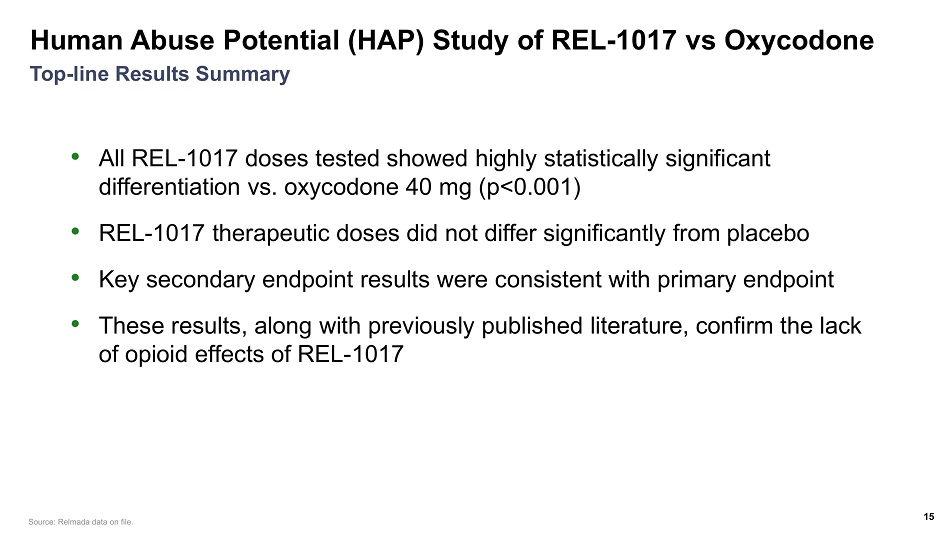
Human Abuse Potential (HAP) Study of REL - 1017 vs Oxycodone • All REL - 1017 doses tested showed highly statistically significant differentiation vs. oxycodone 40 mg (p<0.001) • REL - 1017 therapeutic doses did not differ significantly from placebo • Key secondary endpoint results were consistent with primary endpoint • These results, along with previously published literature, confirm the lack of opioid effects of REL - 1017 15 Top - line Results Summary Source: Relmada data on file.
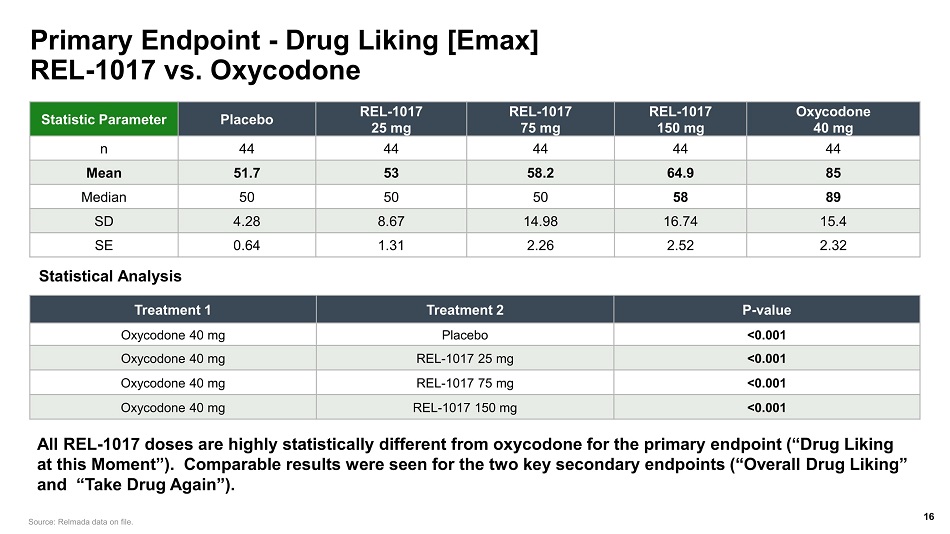
Primary Endpoint - Drug Liking [Emax] REL - 1017 vs. Oxycodone Statistic Parameter Placebo REL - 1017 25 mg REL - 1017 75 mg REL - 1017 150 mg Oxycodone 40 mg n 44 44 44 44 44 Mean 51.7 53 58.2 64.9 85 Median 50 50 50 58 89 SD 4.28 8.67 14.98 16.74 15.4 SE 0.64 1.31 2.26 2.52 2.32 Treatment 1 Treatment 2 P - value Oxycodone 40 mg Placebo <0.001 Oxycodone 40 mg REL - 1017 25 mg <0.001 Oxycodone 40 mg REL - 1017 75 mg <0.001 Oxycodone 40 mg REL - 1017 150 mg <0.001 Statistical Analysis Source: Relmada data on file. All REL - 1017 doses are highly statistically different from oxycodone for the primary endpoint (“Drug Liking at this Moment”). Comparable results were seen for the two key secondary endpoints (“Overall Drug Liking” and “Take Drug Again”). 16
RELIANCE: The Phase 3 Program for REL - 1017
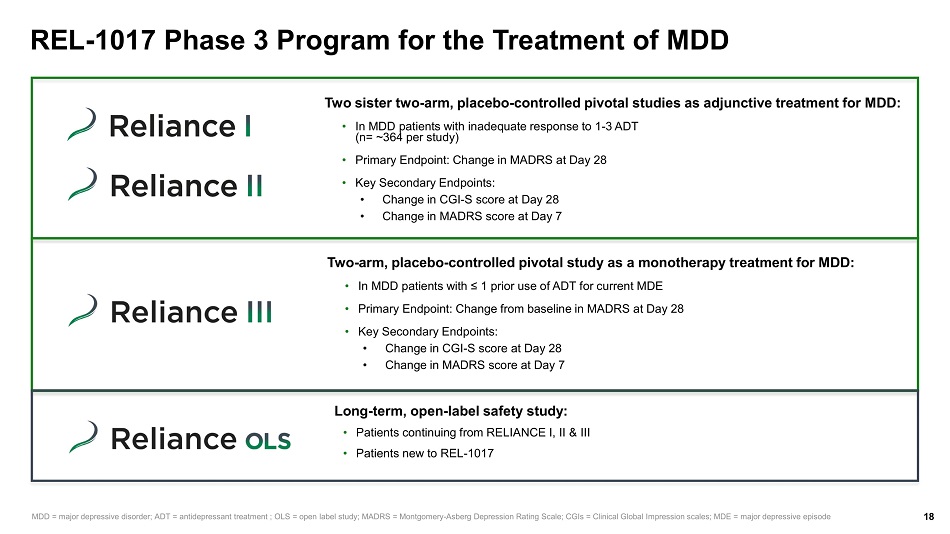
REL - 1017 Phase 3 Program for the Treatment of MDD 18 Two sister two - arm, placebo - controlled pivotal studies as adjunctive treatment for MDD: • In MDD patients with inadequate response to 1 - 3 ADT (n= ~364 per study) • Primary Endpoint: Change in MADRS at Day 28 • Key Secondary Endpoints: • Change in CGI - S score at Day 28 • Change in MADRS score at Day 7 MDD = major depressive disorder; ADT = antidepressant treatment ; OLS = open label study; MADRS = Montgomery - Asberg Depression Rating Scale; CGIs = Clinical Global Impression scales; MDE = major depressive episode Two - arm, placebo - controlled pivotal study as a monotherapy treatment for MDD: • In MDD patients with ≤ 1 prior use of ADT for current MDE • Primary Endpoint: Change from baseline in MADRS at Day 28 • Key Secondary Endpoints: • Change in CGI - S score at Day 28 • Change in MADRS score at Day 7 Long - term, open - label safety study: • Patients continuing from RELIANCE I, II & III • Patients new to REL - 1017
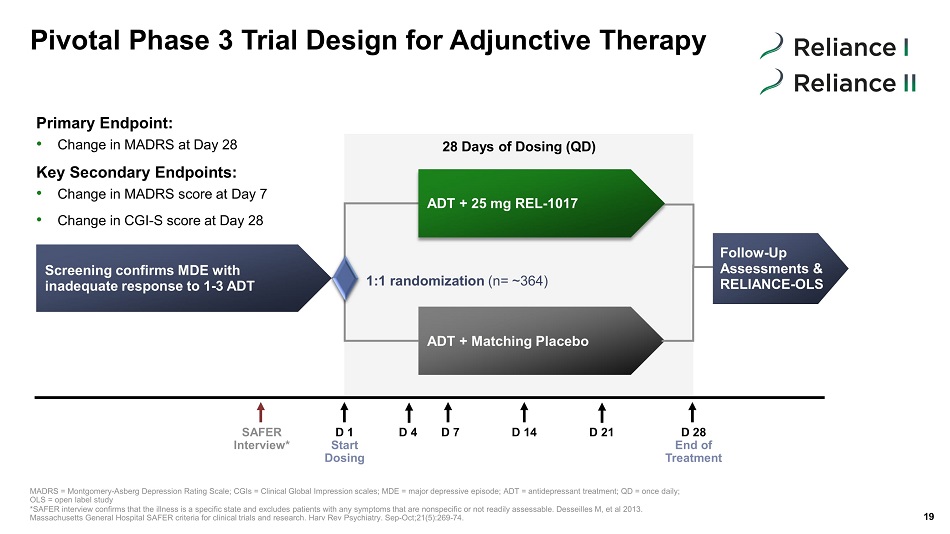
Screening confirms MDE with inadequate response to 1 - 3 ADT ADT + 25 mg REL - 1017 ADT + Matching Placebo Pivotal Phase 3 Trial Design for Adjunctive Therapy 19 1:1 randomization (n= ~ 364 ) 28 Days of Dosing (QD) Follow - Up Assessments & RELIANCE - OLS SAFER Interview* D 1 Start Dosing D 4 D 7 D 14 D 28 End of Treatment D 21 MADRS = Montgomery - Asberg Depression Rating Scale; CGIs = Clinical Global Impression scales; MDE = major depressive episode; ADT = antidepressant treat me nt; QD = once daily; OLS = open label study *SAFER interview confirms that the illness is a specific state and excludes patients with any symptoms that are nonspecific o r n ot readily assessable. Desseilles M, et al 2013. Massachusetts General Hospital SAFER criteria for clinical trials and research. Harv Rev Psychiatry. Sep - Oct;21(5):269 - 74. Primary Endpoint: • Change in MADRS at Day 28 Key Secondary Endpoints: • Change in MADRS score at Day 7 • Change in CGI - S score at Day 28
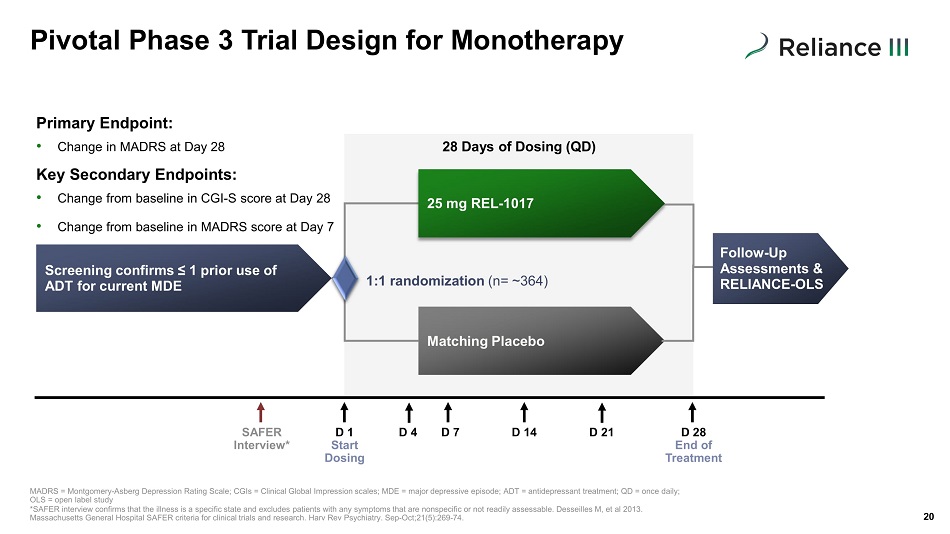
Screening confirms ≤ 1 prior use of ADT for current MDE 25 mg REL - 1017 Matching Placebo Pivotal Phase 3 Trial Design for Monotherapy 20 Primary Endpoint: • Change in MADRS at Day 28 Key Secondary Endpoints: • Change from baseline in CGI - S score at Day 28 • Change from baseline in MADRS score at Day 7 1:1 randomization (n= ~ 364 ) 28 Days of Dosing (QD) Follow - Up Assessments & RELIANCE - OLS SAFER Interview* D 1 Start Dosing D 4 D 7 D 14 D 28 End of Treatment D 21 MADRS = Montgomery - Asberg Depression Rating Scale; CGIs = Clinical Global Impression scales; MDE = major depressive episode; ADT = antidepressant treat me nt; QD = once daily; OLS = open label study *SAFER interview confirms that the illness is a specific state and excludes patients with any symptoms that are nonspecific o r n ot readily assessable. Desseilles M, et al 2013. Massachusetts General Hospital SAFER criteria for clinical trials and research. Harv Rev Psychiatry. Sep - Oct;21(5):269 - 74.
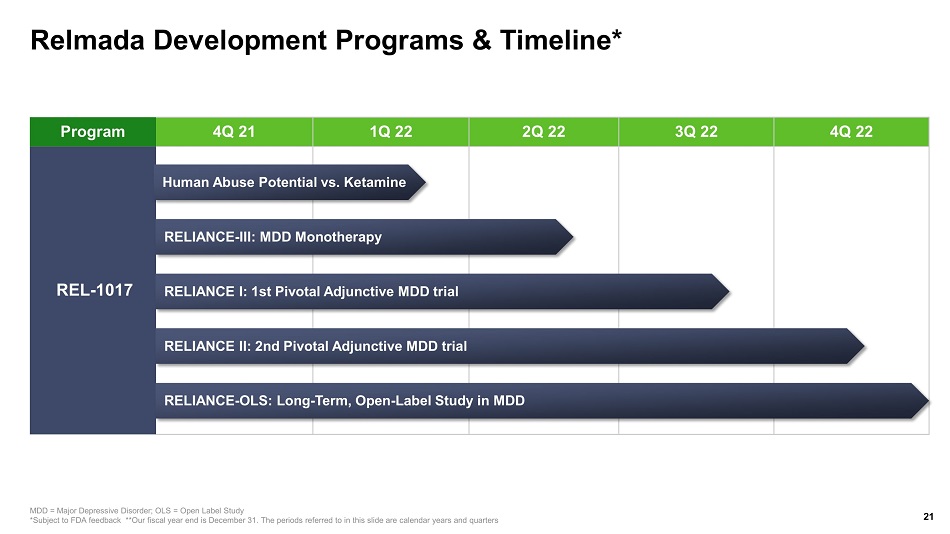
Relmada Development Programs & Timeline* 21 MDD = Major Depressive Disorder; OLS = Open Label Study *Subject to FDA feedback **Our fiscal year end is December 31. The periods referred to in this slide are calendar years and qua rters Program 4Q 21 1Q 22 2Q 22 3Q 22 4Q 22 REL - 1017 RELIANCE - III: MDD Monotherapy RELIANCE I: 1st Pivotal Adjunctive MDD trial RELIANCE II: 2nd Pivotal Adjunctive MDD trial RELIANCE - OLS: Long - Term, Open - Label Study in MDD Human Abuse Potential vs. Ketamine

Novel Psilocybin and Derivatives

Novel Psilocybin and Derivates • July 2021 announcement of licensing agreement: development and commercial rights to a novel psilocybin and derivatives program • Complementary focus: potential therapies for neurodegenerative conditions • Synergy in neuroplasticity: Expands R&D portfolio while building on expertise in neural plasticity, specifically the neuroplastogen mechanism of action • Like REL - 1017, will build upon Relmada’s expertise on neuroplasticity • Development and commercial rights in all ex - Asia territories, including the U.S. and Europe 23 Rights to Co - Develop and Commercialize

Corporate Information
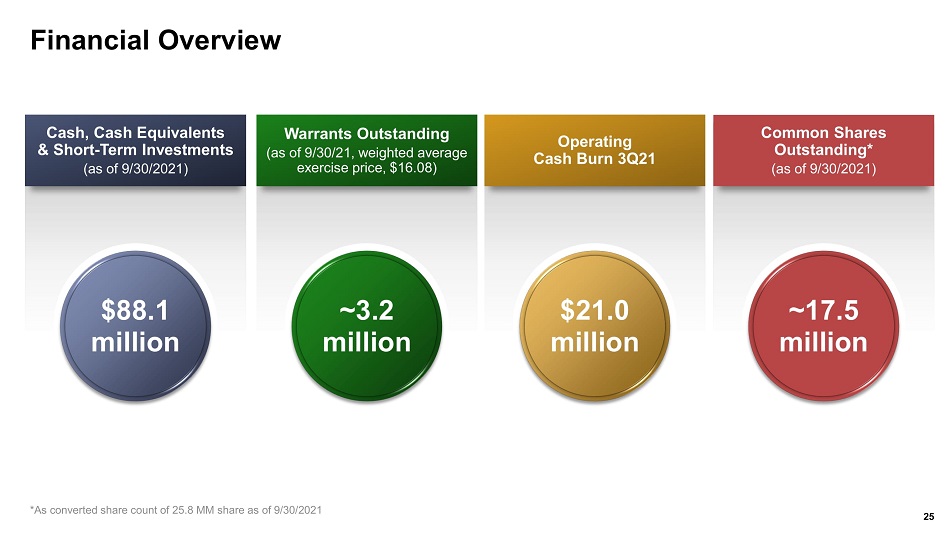
Financial Overview Cash, Cash Equivalents & Short - Term Investments (as of 9/30/2021) $88.1 million Warrants Outstanding (as of 9/30/21, weighted average exercise price, $16.08) ~3.2 million Operating Cash Burn 3Q21 $ 21.0 million Common Shares Outstanding* (as of 9 /30/2021) ~17.5 million 25 *As converted share count of 25.8 MM share as of 9/30/2021
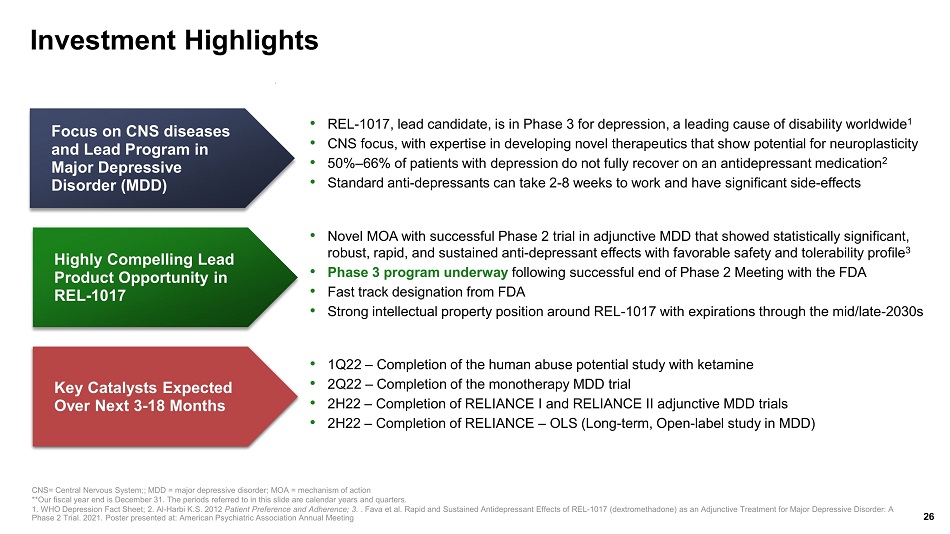
Investment Highlights Highly Compelling Lead Product Opportunity in REL - 1017 Key Catalysts Expected Over Next 3 - 18 Months Focus on CNS diseases and Lead Program in Major Depressive Disorder (MDD) CNS= Central Nervous System;; MDD = major depressive disorder; MOA = mechanism of action **Our fiscal year end is December 31. The periods referred to in this slide are calendar years and quarters. 1. WHO Depression Fact Sheet; 2. Al - Harbi K.S. 2012 Patient Preference and Adherence; 3. . Fava et al. Rapid and Sustained Antidepressant Effects of REL - 1017 ( dextromethadone ) as an Adjunctive Treatment for Major Depressive Disorder: A Phase 2 Trial. 2021. Poster presented at: American Psychiatric Association Annual Meeting 26 • Novel MOA with successful Phase 2 trial in adjunctive MDD that showed statistically significant, robust, rapid, and sustained anti - depressant effects with favorable safety and tolerability profile 3 • Phase 3 program underway following successful end of Phase 2 Meeting with the FDA • Fast track designation from FDA • Strong intellectual property position around REL - 1017 with expirations through the mid/late - 2030s • REL - 1017, l ead candidate, is in Phase 3 for depression, a leading cause of disability worldwide 1 • CNS focus, with expertise in developing novel therapeutics that show potential for neuroplasticity • 50% – 66% of patients with depression do not fully recover on an antidepressant medication 2 • Standard anti - depressants can take 2 - 8 weeks to work and have significant side - effects • 1Q22 – Completion of the human abuse potential study with ketamine • 2 Q22 – Completion of the monotherapy MDD trial • 2 H22 – Completion of RELIANCE I and RELIANCE II adjunctive MDD trials • 2H22 – Completion of RELIANCE – OLS (Long - term, Open - label study in MDD)