
Exhibit 99.2

Human Abuse Potential (HAP) Study of REL - 1017 vs. Ketamine February 23, 2022 I Nasdaq: RLMD 1

Disclosures Certain statements contained in this presentation or in other documents of Relmada Therapeutics, Inc. (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward - lookin g statements.” These statements can be identified by the fact that they do not relate strictly to historic or current facts. Th ey use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar mea nin g in connection with any discussion of future operating or financial performance or condition. These statements are based upon the cu rrent beliefs and expectations of the Company’s management and are subject to significant risks and uncertainties. Statements regar din g future action, future performance and/or future results, those relating to the timing for completion, and results of, schedul ed or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results ( if any) of the Company’s formulations and products and regulatory filings related to the same may differ from those set forth in the for war d - looking statements. Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward - looking statement. It is not possible to predict or identify all such risks, contingencies and uncertainties. The Company identifies some of these factors in its S ecu rities and Exchange Commission (“SEC”) filings on Forms 10 - K, 10 - Q and 8 - K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties affecting the Company and its business and finan cia l performance. The Company assumes no obligation to update forward - looking statements as circumstances change. Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10 - K, 10 - Q and 8 - K reports. 2

Human Abuse Potential (HAP) Study of REL - 1017 vs Ketamine Introduction Sergio Traversa, Chief Executive Officer of Relmada Study Design & Top - Line Results Jack Henningfield , Ph.D., Vice President, Research, Health Policy, and Abuse Liability at Pinney Associates; former Chief of the Clinical Pharmacology Research Branch, and the Abuse Potential and Biology of Dependence Assessment Section of the National Institute on Drug Abuse (NIDA); four decades experience in HAP assessment beginning at Johns Hopkins School of Medicine and NIDA 3 Top - Line Results – Conference Call & Webcast Agenda

Human Abuse Potential (HAP) Study of REL - 1017 vs. IV Ketamine Summary • All REL - 1017 doses, including 150 mg (6x therapeutic dose and MTD), were statistically different from IV ketamine 0.5 mg/Kg (p<0.05) • All REL - 1017 doses including, 150 mg (6x therapeutic dose and MTD), were statistically equivalent to placebo (p<0.05) • Key secondary endpoint results were consistent with primary endpoint 4 MTD = Maximum tolerated dose; IV = Intravenous, Source: Relmada data on file. Primary endpoint, Drug Liking (Emax) Completer Population
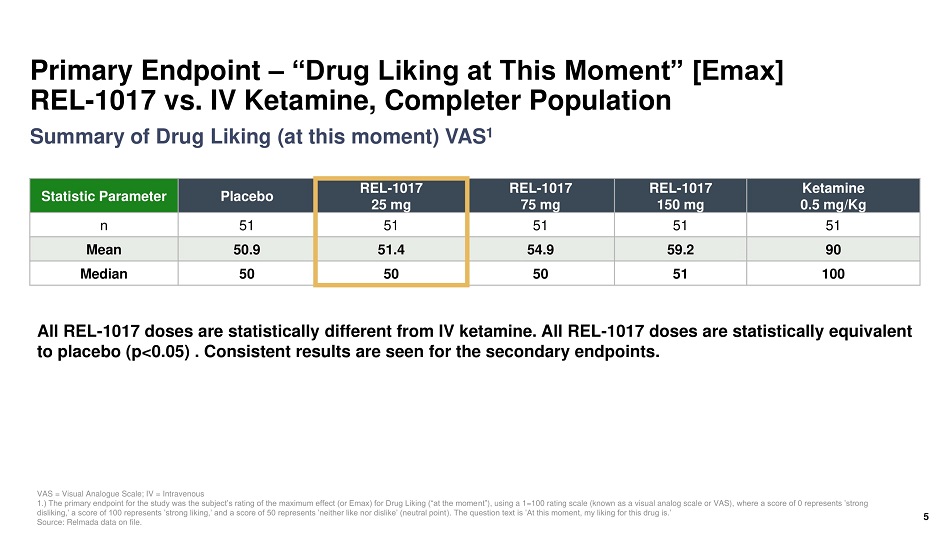
Primary Endpoint – “Drug Liking at This Moment” [Emax] REL - 1017 vs. IV Ketamine, Completer Population Statistic Parameter Placebo REL - 1017 25 mg REL - 1017 75 mg REL - 1017 150 mg Ketamine 0.5 mg/Kg n 51 51 51 51 51 Mean 50.9 51.4 54.9 59.2 90 Median 50 50 50 51 100 VAS = Visual Analogue Scale; IV = Intravenous 1.) The primary endpoint for the study was the subject’s rating of the maximum effect (or Emax) for Drug Liking (“at the mome nt” ), using a 1=100 rating scale (known as a visual analog scale or VAS), where a score of 0 represents ’strong disliking,’ a score of 100 represents ’strong liking,’ and a score of 50 represents ’neither like nor dislike’ (neutral point ). The question text is ’At this moment, my liking for this drug is.’ Source: Relmada data on file. All REL - 1017 doses are statistically different from IV ketamine. All REL - 1017 doses are statistically equivalent to placebo (p<0.05) . Consistent results are seen for the secondary endpoints. 5 Summary of Drug Liking (at this moment) VAS 1
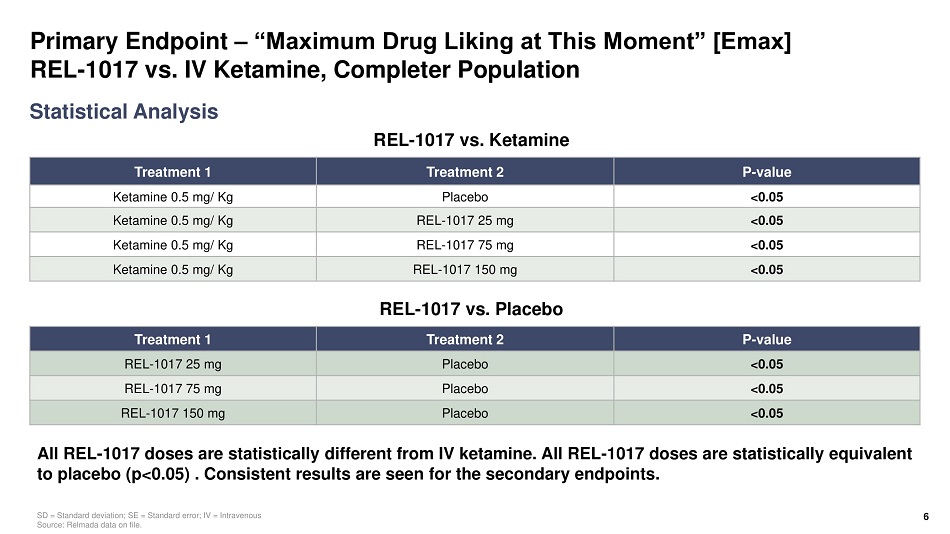
Primary Endpoint – “Maximum Drug Liking at This Moment” [Emax] REL - 1017 vs. IV Ketamine, Completer Population Treatment 1 Treatment 2 P - value Ketamine 0.5 mg/ Kg Placebo <0.05 Ketamine 0.5 mg/ Kg REL - 1017 25 mg <0.05 Ketamine 0.5 mg/ Kg REL - 1017 75 mg <0.05 Ketamine 0.5 mg/ Kg REL - 1017 150 mg <0.05 Statistical Analysis SD = Standard deviation; SE = Standard error; IV = Intravenous Source: Relmada data on file. All REL - 1017 doses are statistically different from IV ketamine. All REL - 1017 doses are statistically equivalent to placebo (p<0.05) . Consistent results are seen for the secondary endpoints. 6 Treatment 1 Treatment 2 P - value REL - 1017 25 mg Placebo <0.05 REL - 1017 75 mg Placebo <0.05 REL - 1017 150 mg Placebo <0.05 REL - 1017 vs. Ketamine REL - 1017 vs. Placebo
US DEA July 2019 Statement on esmethadone 7 “The d - isomer lacks significant respiratory depressant action and addiction liability.. .” - DEA Statement July 2019 1 1.US DEA Statement on Methadone 2019 https:// www.deadiversion.usdoj.gov / drug_chem_info /methadone/ methadone.pdf#search =methadone

Q&A

Thank You