
Exhibit 99.2

Reliance I Topline REL - 1017 for Major Depressive Disorder (MDD) Dec 07, 2022 Nasdaq: RLMD EXHIBIT 99.2

©2022 Relmada All rights reserved Disclosures Certain statements contained in this presentation or in other documents of Relmada Therapeutics, Inc. (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward - looking statements.” These statements can be identified by the fact that they do not relate strictly to historic or current facts. They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition. These statements are based upon the current beliefs and expectations of the Company’s management and are subject to significant risks and uncertainties. Statements regarding future action, future performance and/or future results, those relating to the timing for completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formulations and products and regulatory filings related to the same may differ from those set forth in the forward - looking statements. Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate. Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward - looking statement. It is not possible to predict or identify all such risks, contingencies and uncertainties. The Company identifies some of these factors in its Securities and Exchange Commission (“SEC”) filings on Forms 10 - K, 10 - Q and 8 - K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties affecting the Company and its business and financial performance. The Company assumes no obligation to update forward - looking statements as circumstances change. Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10 - K, 10 - Q and 8 - K reports. 2

Topline Data Reliance I
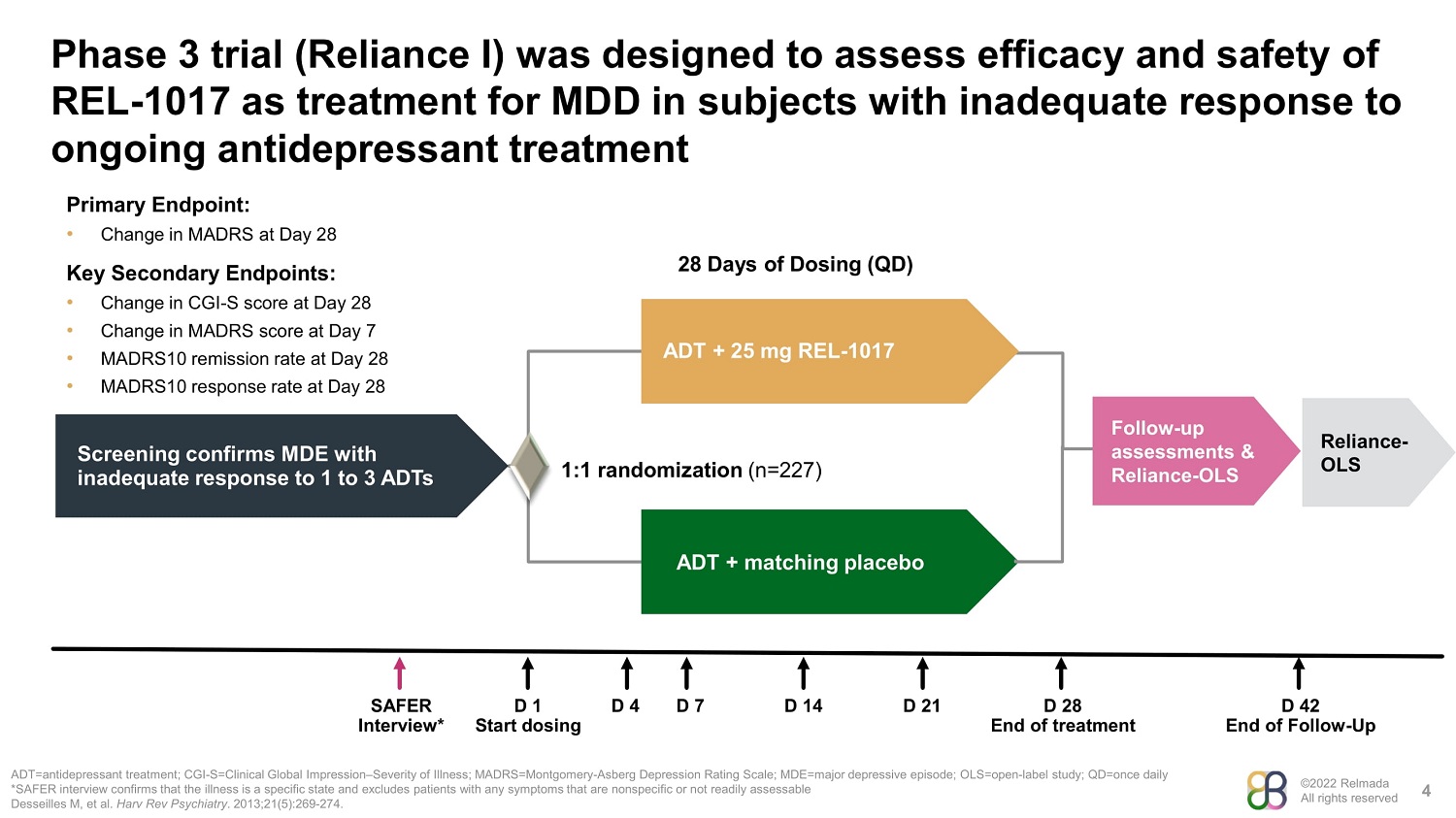
©2022 Relmada All rights reserved Phase 3 trial (Reliance I) was designed to assess efficacy and safety of REL - 1017 as treatment for MDD in subjects with inadequate response to ongoing antidepressant treatment 4 ADT=antidepressant treatment; CGI - S=Clinical Global Impression – Severity of Illness; MADRS=Montgomery - Asberg Depression Rating Scale; MDE=major depressive episode; OLS=open - label study; QD=once daily *SAFER interview confirms that the illness is a specific state and excludes patients with any symptoms that are nonspecific or not readily assessable Desseilles M, et al. Harv Rev Psychiatry . 2013;21(5):269 - 274. Screening confirms MDE with inadequate response to 1 to 3 ADTs ADT + 25 mg REL - 1017 ADT + matching placebo 1:1 randomization (n=227) 28 Days of Dosing (QD) Follow - up assessments & Reliance - OLS SAFER D 1 D 4 D 7 D 14 D 21 D 28 D 42 Interview* Start dosing End of treatment End of Follow - Up Primary Endpoint: • Change in MADRS at Day 28 Key Secondary Endpoints: • Change in CGI - S score at Day 28 • Change in MADRS score at Day 7 • MADRS10 remission rate at Day 28 • MADRS10 response rate at Day 28 Reliance - OLS
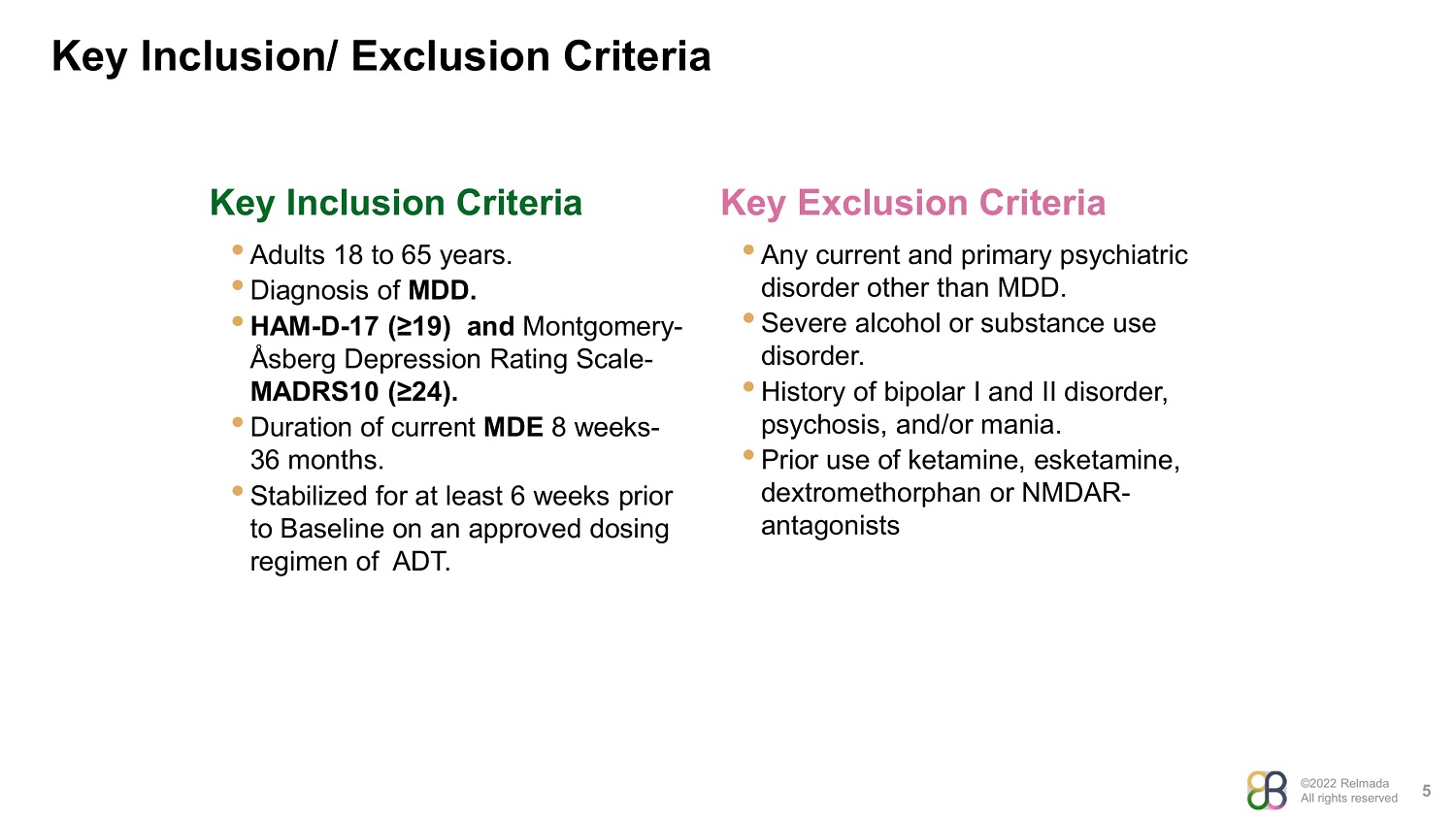
©2022 Relmada All rights reserved Key Inclusion/ Exclusion Criteria 5 Key Inclusion Criteria Key Exclusion Criteria • Adults 18 to 65 years. • Diagnosis of MDD. • HAM - D - 17 (≥19) and Montgomery - Åsberg Depression Rating Scale - MADRS10 (≥24). • Duration of current MDE 8 weeks - 36 months . • Stabilized for at least 6 weeks prior to Baseline on an approved dosing regimen of ADT . • Any current and primary psychiatric disorder other than MDD. • Severe alcohol or substance use disorder. • History of bipolar I and II disorder, psychosis, and/or mania. • Prior use of ketamine, esketamine, dextromethorphan or NMDAR - antagonists
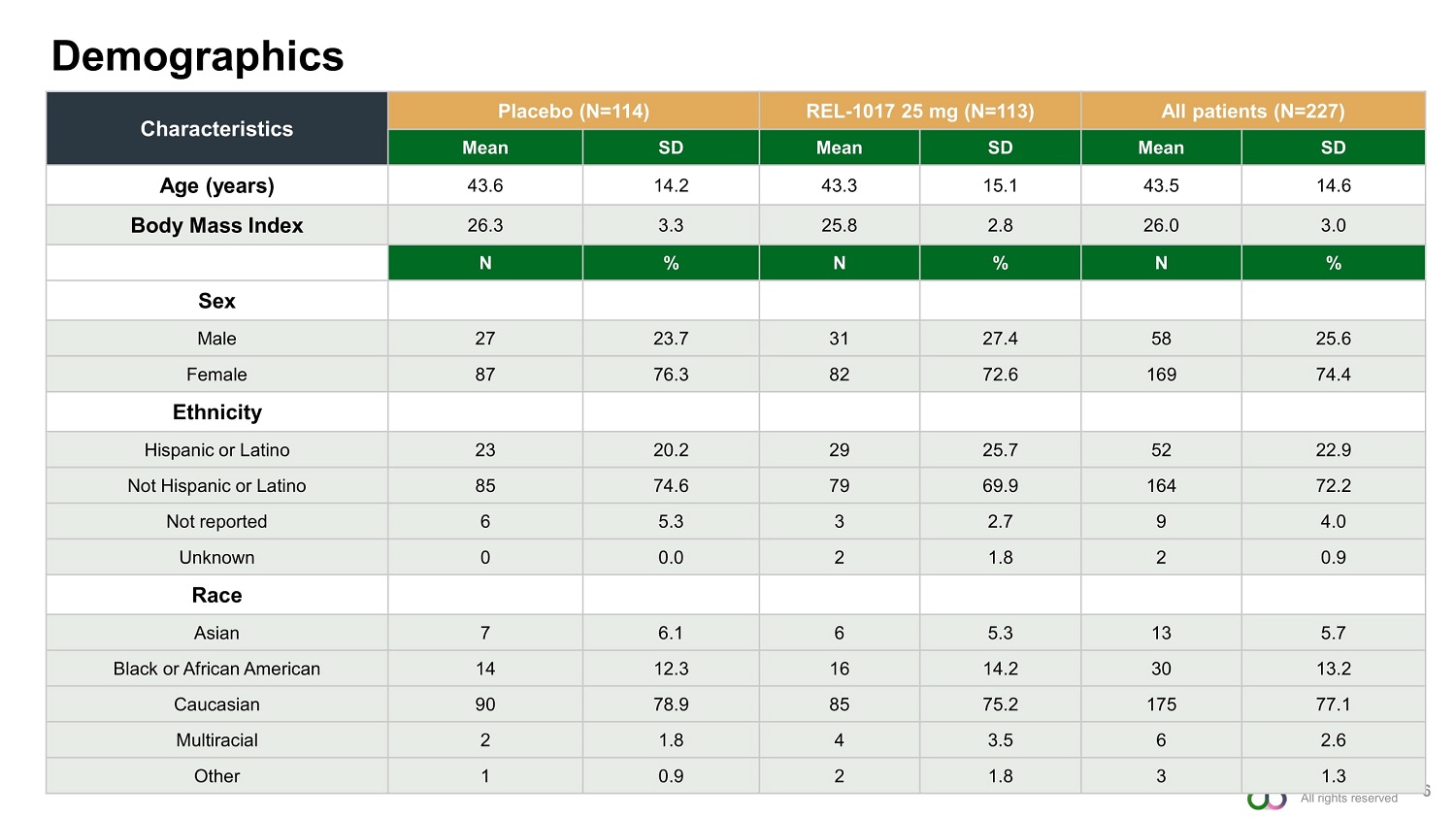
©2022 Relmada Demographics 6 Characteristics Placebo (N=114) REL - 1017 25 mg (N=113) All patients (N=227) Mean SD Mean SD Mean SD Age (years) 43.6 14.2 43.3 15.1 43.5 14.6 Body Mass Index 26.3 3.3 25.8 2.8 26.0 3.0 N % N % N % Sex Male 27 23.7 31 27.4 58 25.6 Female 87 76.3 82 72.6 169 74.4 Ethnicity Hispanic or Latino 23 20.2 29 25.7 52 22.9 Not Hispanic or Latino 85 74.6 79 69.9 164 72.2 Not reported 6 5.3 3 2.7 9 4.0 Unknown 0 0.0 2 1.8 2 0.9 Race Asian 7 6.1 6 5.3 13 5.7 Black or African American 14 12.3 16 14.2 30 13.2 Caucasian 90 78.9 85 75.2 175 77.1 Multiracial 2 1.8 4 3.5 6 2.6 Other 1 0.9 2 1.8 3 1.3 All rights reserved
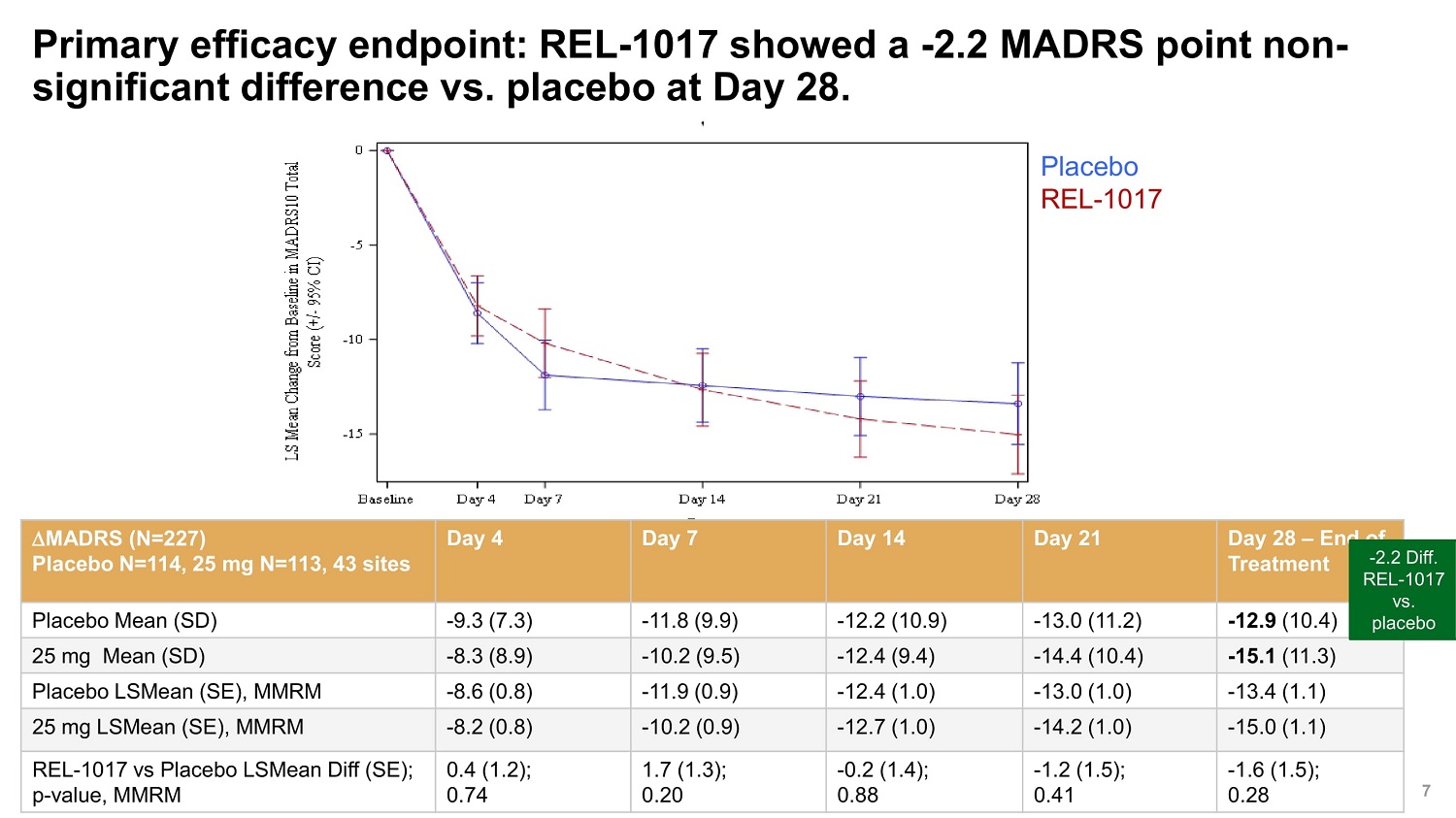
©2022 Relmada All rights reserved Primary efficacy endpoint: REL - 1017 showed a - 2.2 MADRS point non - significant difference vs. placebo at Day 28. 7 MADRS (N=227) Placebo N=114, 25 mg N=113, 43 sites Day 4 Day 7 Day 14 Day 21 Day 28 – End of Treatment - 2.2 REL - vs Placebo Mean (SD) - 9.3 (7.3) - 11.8 (9.9) - 12.2 (10.9) - 13.0 (11.2) - 12.9 (10.4) plac 25 mg Mean (SD) - 8.3 (8.9) - 10.2 (9.5) - 12.4 (9.4) - 14.4 (10.4) - 15.1 (11.3) Placebo LSMean (SE), MMRM - 8.6 (0.8) - 11.9 (0.9) - 12.4 (1.0) - 13.0 (1.0) - 13.4 (1.1) 25 mg LSMean (SE), MMRM - 8.2 (0.8) - 10.2 (0.9) - 12.7 (1.0) - 14.2 (1.0) - 15.0 (1.1) REL - 1017 vs Placebo LSMean Diff (SE); p - value, MMRM 0.4 (1.2); 0.74 1.7 (1.3); 0.20 - 0.2 (1.4); 0.88 - 1.2 (1.5); 0.41 - 1.6 (1.5); 0.28 Diff. 1017 . ebo Placebo REL - 1017
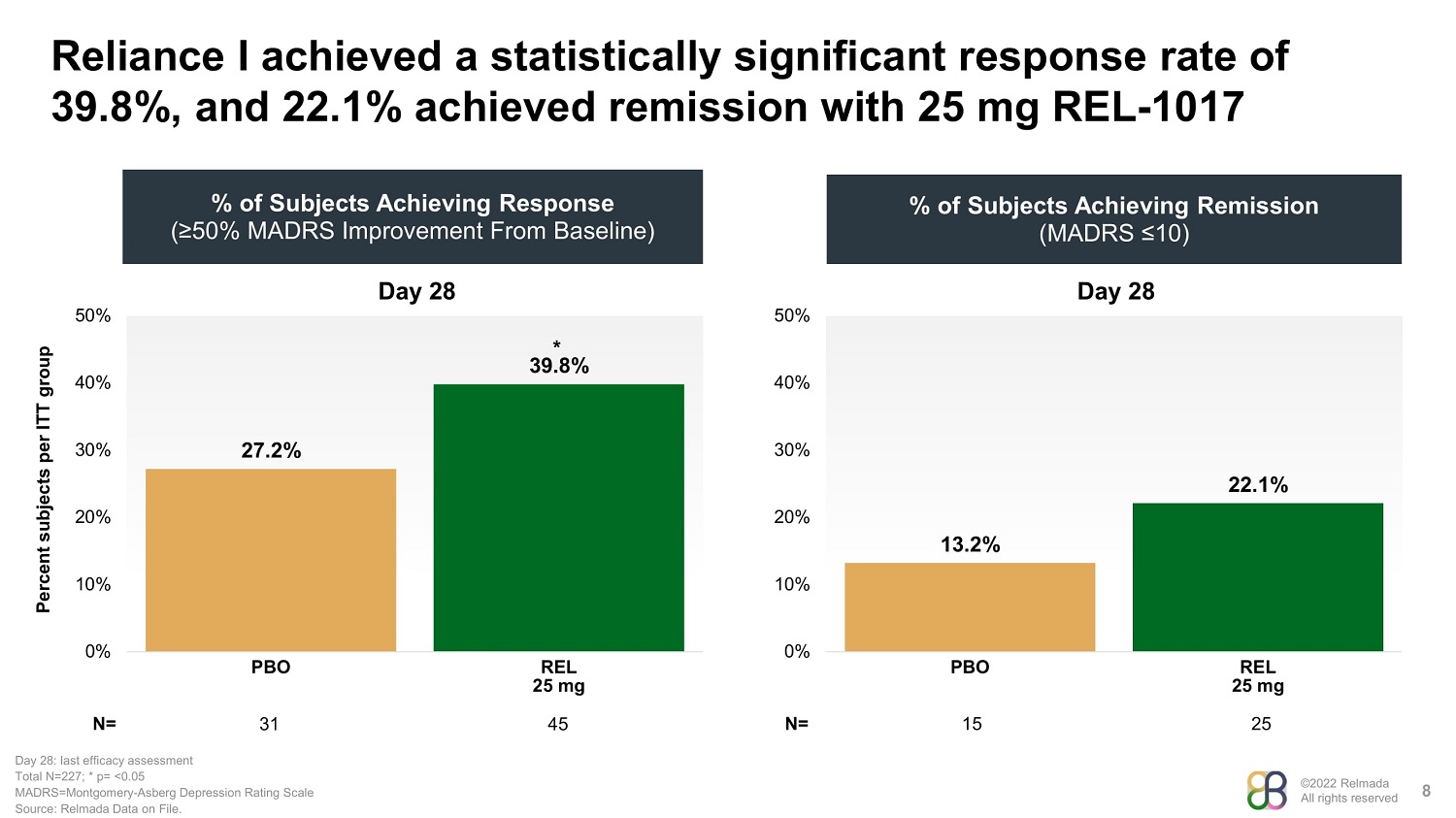
©2022 Relmada All rights reserved Reliance I achieved a statistically significant response rate of 39.8%, and 22.1% achieved remission with 25 mg REL - 1017 8 % of Subjects Achieving Remission (MADRS ≤10) Percent subjects per ITT group % of Subjects Achieving Response (≥50% MADRS Improvement From Baseline) N= 31 Day 28: last efficacy assessment Total N=227; * p= <0.05 MADRS=Montgomery - Asberg Depression Rating Scale Source: Relmada Data on File. Day 28 27.2% 39.8% 0% 10% 20% 30% 40% 50% PBO REL 25 mg 45 Day 28 13.2% 22.1% 0% 10% 20% 30% 40% 50% PBO REL 25 mg 15 25 N= *
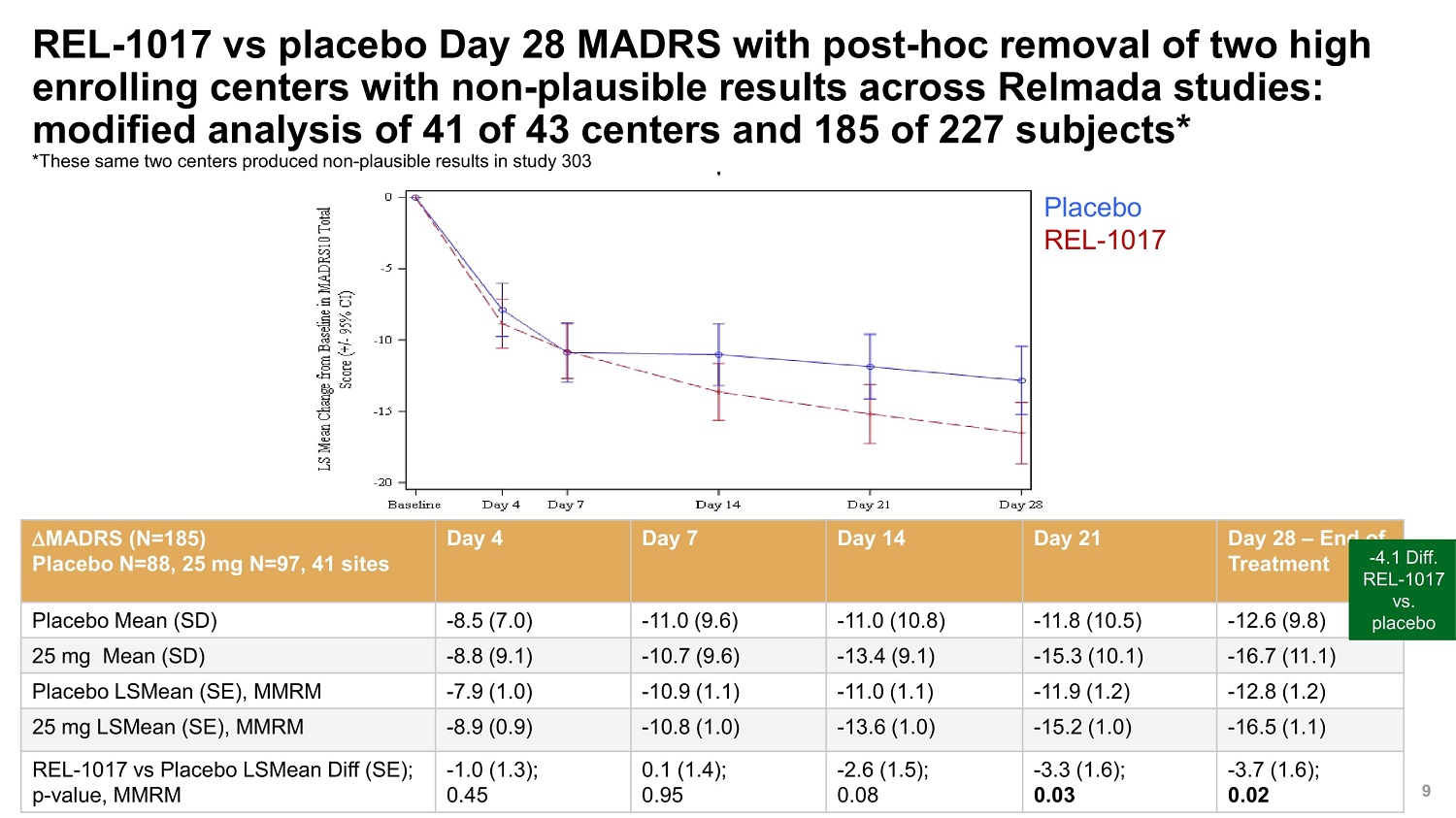
©2022 Relmada All rights reserved REL - 1017 vs placebo Day 28 MADRS with post - hoc removal of two high enrolling centers with non - plausible results across Relmada studies: modified analysis of 41 of 43 centers and 185 of 227 subjects* *These same two centers produced non - plausible results in study 303 9 MADRS (N=185) Placebo N=88, 25 mg N=97, 41 sites Day 4 Day 7 Day 14 Day 21 Day 28 – End of Treatment - 4.1 REL - vs Placebo Mean (SD) - 8.5 (7.0) - 11.0 (9.6) - 11.0 (10.8) - 11.8 (10.5) - 12.6 (9.8) plac 25 mg Mean (SD) - 8.8 (9.1) - 10.7 (9.6) - 13.4 (9.1) - 15.3 (10.1) - 16.7 (11.1) Placebo LSMean (SE), MMRM - 7.9 (1.0) - 10.9 (1.1) - 11.0 (1.1) - 11.9 (1.2) - 12.8 (1.2) 25 mg LSMean (SE), MMRM - 8.9 (0.9) - 10.8 (1.0) - 13.6 (1.0) - 15.2 (1.0) - 16.5 (1.1) REL - 1017 vs Placebo LSMean Diff (SE); p - value, MMRM - 1.0 (1.3); 0.45 0.1 (1.4); 0.95 - 2.6 (1.5); 0.08 - 3.3 (1.6); 0.03 - 3.7 (1.6); 0.02 Diff. 1017 . ebo Placebo REL - 1017
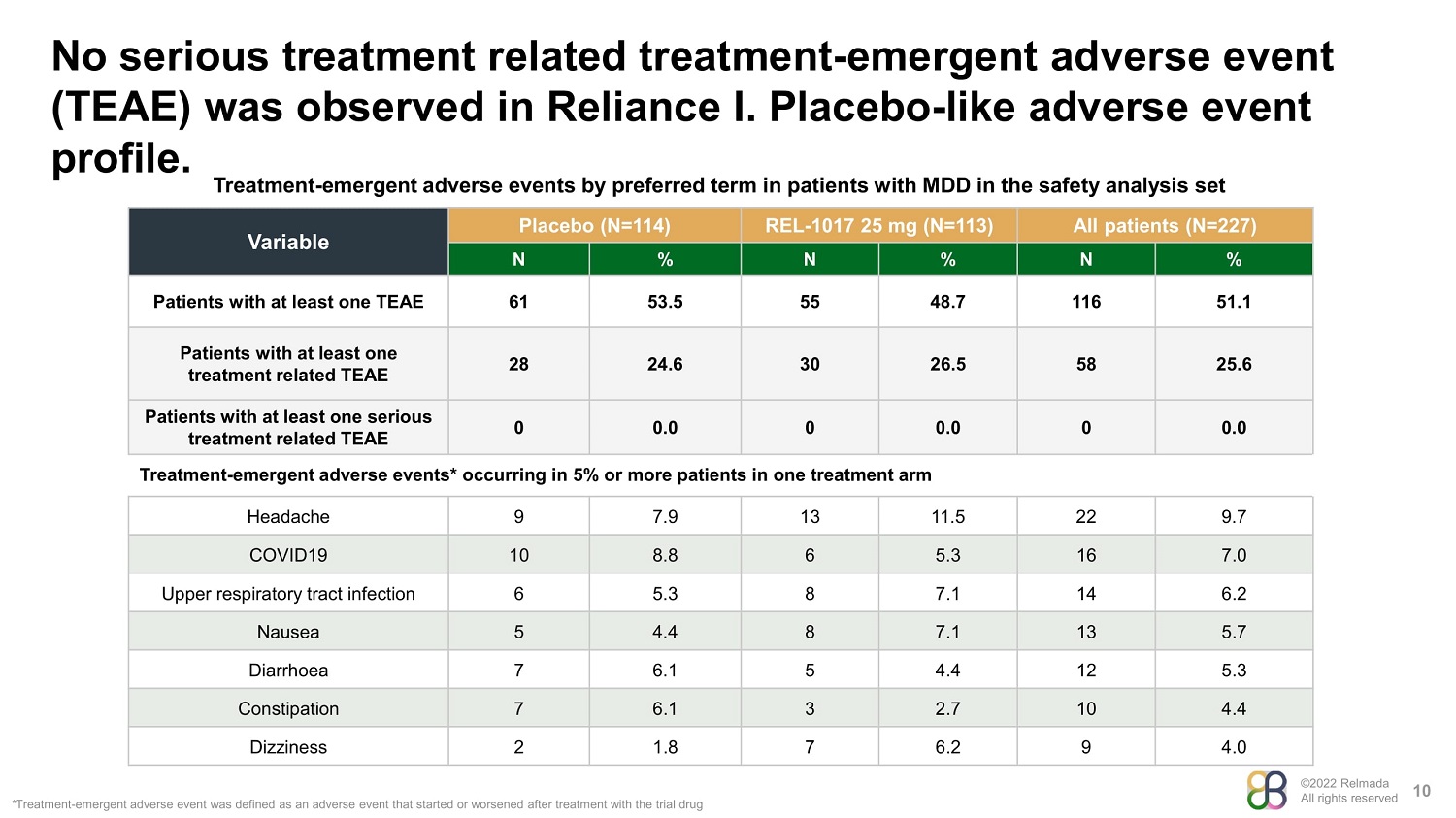
©2022 Relmada All rights reserved No serious treatment related treatment - emergent adverse event (TEAE) was observed in Reliance I. Placebo - like adverse event profile. 10 Treatment - emergent adverse events by preferred term in patients with MDD in the safety analysis set Variable Placebo (N=114) REL - 1017 25 mg (N=113) All patients (N=227) N % N % N % Patients with at least one TEAE 61 53.5 55 48.7 116 51.1 Patients with at least one treatment related TEAE 28 24.6 30 26.5 58 25.6 Patients with at least one serious treatment related TEAE 0 0.0 0 0.0 0 0.0 Treatment - emergent adverse events* occurring in 5% or more patients in one treatment arm Headache 9 7.9 13 11.5 22 9.7 COVID19 10 8.8 6 5.3 16 7.0 Upper respiratory tract infection 6 5.3 8 7.1 14 6.2 Nausea 5 4.4 8 7.1 13 5.7 Diarrhoea 7 6.1 5 4.4 12 5.3 Constipation 7 6.1 3 2.7 10 4.4 Dizziness 2 1.8 7 6.2 9 4.0 * Treatment - emergent adverse event was defined as an adverse event that started or worsened after treatment with the trial drug
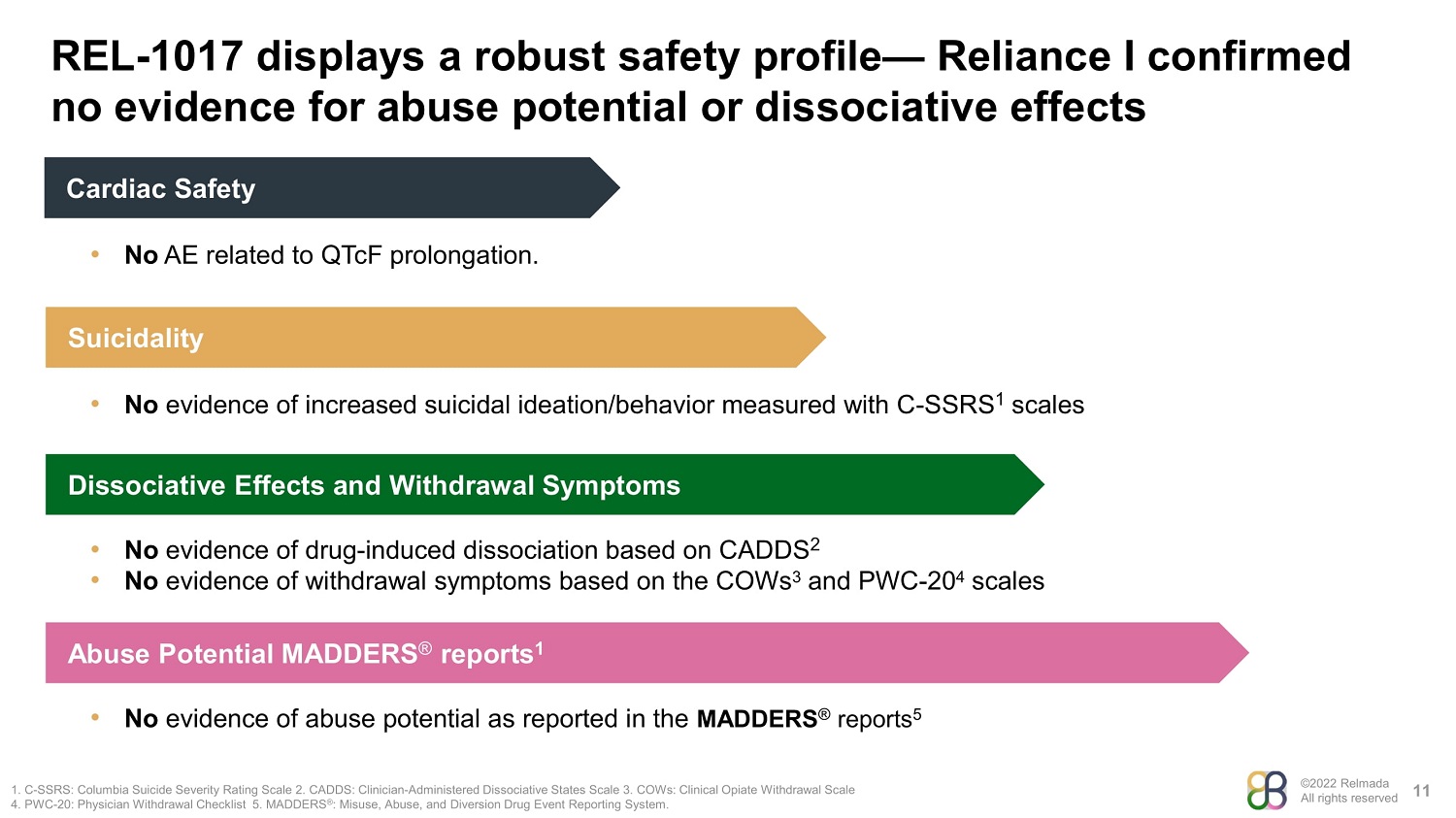
©2022 Relmada All rights reserved REL - 1017 displays a robust safety profile — Reliance I confirmed no evidence for abuse potential or dissociative effects 11 1. C - SSRS: Columbia Suicide Severity Rating Scale 2. CADDS: Clinician - Administered Dissociative States Scale 3. COWs: Clinical Opiate Withdrawal Scale 4. PWC - 20: Physician Withdrawal Checklist 5. MADDERS ® : Misuse, Abuse, and Diversion Drug Event Reporting System. Dissociative Effects and Withdrawal Symptoms • No evidence of drug - induced dissociation based on CADDS 2 • No evidence of withdrawal symptoms based on the COWs 3 and PWC - 20 4 scales Abuse Potential MADDERS ® reports 1 • No evidence of abuse potential as reported in the MADDERS ® reports 5 Cardiac Safety • No AE related to QTcF prolongation. Suicidality • No evidence of increased suicidal ideation/behavior measured with C - SSRS 1 scales
©2022 Relmada All rights reserved Summary 12 Efficacy • REL - 1017 shows a - 2.2 MADRS point non - significant difference to placebo • Statistically significant MADRS response rate of REL - 1017 compared to placebo • A post - hoc analysis with the exclusion of 2 non - plausible high enrolling centers, previously identified in Reliance III, results in a statistically significant 4.1 difference from placebo (p=0.02) Safety • Placebo - like adverse event profile. • No evidence for abuse potential or dissociative effects

Thank you!