
Exhibit 99.1

Targeting Major Advances in the Treatment of CNS Disorders May 15 th , 2023

©202 3 Relmada - All rights reserve d Disclosures 2 Certain statements contained in this presentation or in other documents of Relmada Therapeutics, Inc. (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward - lookin g statements.” These statements can be identified by the fact that they do not relate strictly to historic or current facts. Th ey use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition. These statements are based upon the current be lie fs and expectations of the Company’s management and are subject to significant risks and uncertainties. Statements regarding future act ion, future performance and/or future results, those relating to the timing for completion, and results of, scheduled or additiona l c linical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s fo rmu lations and products and regulatory filings related to the same may differ from those set forth in the forward - looking statements. Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances ca n be given that such sales levels will be achieved , if at all, or that such market size estimates will prove accurate. Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions in vestors that actual results may differ materially from those expressed or implied in any forward - looking statement. It is not possible to predict or identify all such risks, contingencies and uncertainties. The Company identifies some of these factors in its S ecu rities and Exchange Commission (“SEC”) filings on Forms 10 - K, 10 - Q and 8 - K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties affecting the Company and its business and financial p erf ormance. The Company assumes no obligation to update forward - looking statements as circumstances change. Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10 - K, 10 - Q and 8 - K reports.
©202 3 Relmada - All rights reserve d Highly experienced clinical team with a successful track record advancing CNS programs through NDA approval Significant lessons learned from post - hoc analyses of Phase 3 data provide confidence in the path forward to ongoing and future studies Efficacy and safety profile of REL - 1017 potentially address limitations of current MDD treatments options Compelling Phase 2 data indicating the robust therapeutic effect of REL - 1017 as an adjunctive treatment of MDD Investment highlights 3 CNS= Central Nervous System **Our fiscal year end is December 31. The periods referred to in this slide are calendar years and quarters. CNS focused, with a novel MOA lead program REL - 1017 currently in Phase 3 for Major Depressive Disorder (MDD)

©202 3 Relmada - All rights reserve d 4 Major Depressive Disorder and REL - 1017’s Novel Mechanism of Action
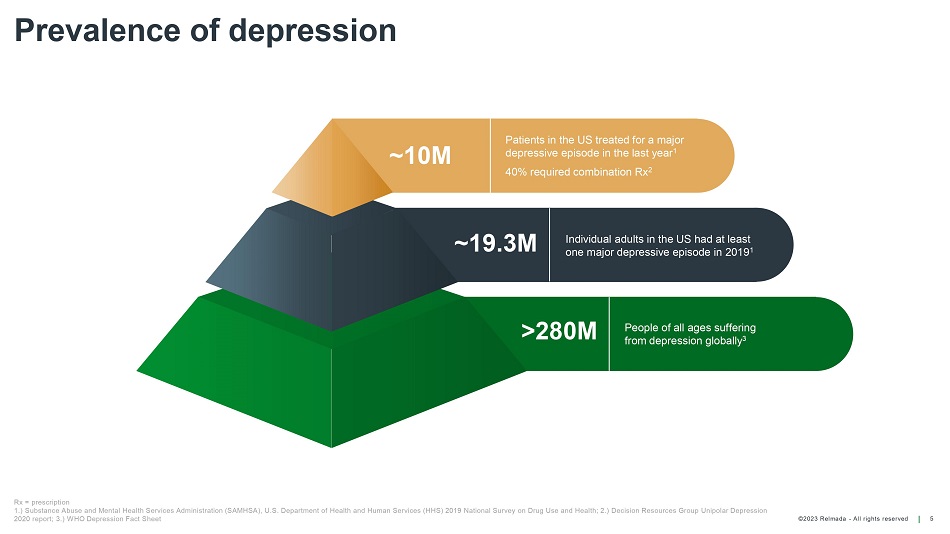
©202 3 Relmada - All rights reserve d Prevalence of depression 5 ~10M Patients in the US treated for a major depressive episode in the last year 1 40% required combination Rx 2 ~19.3M Individual adults in the US had at least one major depressive episode in 2019 1 >280M People of all ages suffer ing from depression globally 3 Rx = prescription 1.) Substance Abuse and Mental Health Services Administration (SAMHSA), U.S. Department of Health and Human Services (HHS) 20 19 National Survey on Drug Use and Health; 2.) Decision Resources Group Unipolar Depression 2020 report; 3.) WHO Depression Fact Sheet

©202 3 Relmada - All rights reserve d Limitations of current treatments for MDD 6 MDD = major depressive disorder 1.) Trivedi MH, et al. Am J Psychiatry. 2006;163:28 - 40; 2.) Ashton AK, et al. Curr Ther Res. 2005;66(2):97 - 106; 3.) US Prescribing Information, brexpiprazole , quetiapine, aripiprazole Limited efficacy Slow onset of action Safety and tolerability challenges ~65% MDD patients do not respond to first antidepressant treatment 1 Side effects of atypical antipsychotics approved as adjunctive treatments for MDD include tardive dyskinesia, metabolic syndrome, cognitive impairment and stroke 3 Standard antidepressants, even when effective, may take up to 8 weeks to reach efficacy 2
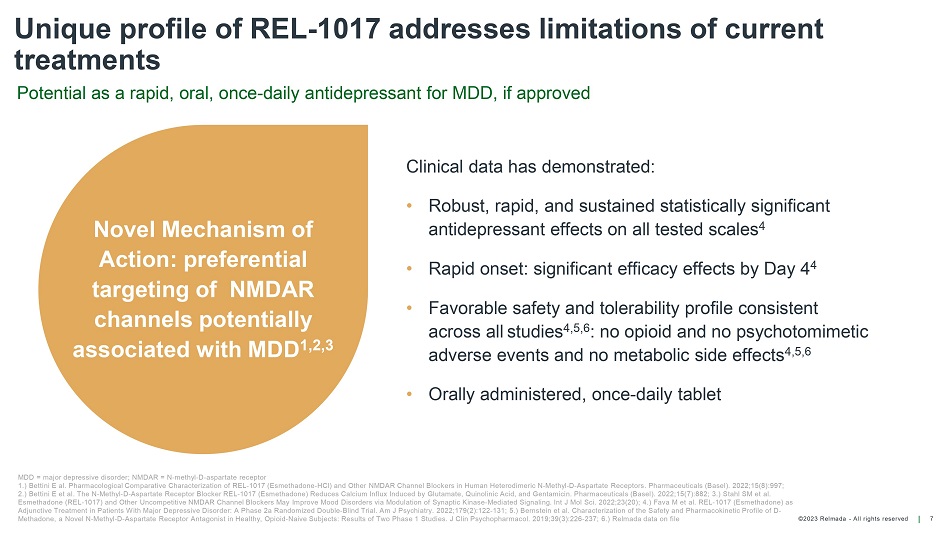
©202 3 Relmada - All rights reserve d Unique profile of REL - 1017 addresses limitations of current treatments 7 Potential as a rapid, oral, once - daily antidepressant for MDD, if approved MDD = major depressive disorder; NMDAR = N - methyl - D - aspartate receptor 1.) Bettini E al. Pharmacological Comparative Characterization of REL - 1017 (Esmethadone - HCl) and Other NMDAR Channel Blockers in Human Heterodimeric N - Methyl - D - Aspartate Receptors. Pharmaceuticals (Basel). 2022;15(8):997; 2.) Bettini E et al. The N - Methyl - D - Aspartate Receptor Blocker REL - 1017 (Esmethadone) Reduces Calcium Influx Induced by Glutamat e, Quinolinic Acid, and Gentamicin. Pharmaceuticals (Basel). 2022;15(7):882; 3.) Stahl SM et al. Esmethadone (REL - 1017) and Other Uncompetitive NMDAR Channel Blockers May Improve Mood Disorders via Modulation of Synaptic Kina se - Mediated Signaling. Int J Mol Sci. 2022;23(20); 4.) Fava M et al. REL - 1017 (Esmethadone) as Adjunctive Treatment in Patients With Major Depressive Disorder: A Phase 2a Randomized Double - Blind Trial. Am J Psychiatry. 2022 ;179(2):122 - 131; 5.) Bernstein et al. Characterization of the Safety and Pharmacokinetic Profile of D - Methadone, a Novel N - Methyl - D - Aspartate Receptor Antagonist in Healthy, Opioid - Naive Subjects: Results of Two Phase 1 Studies. J Clin Psychopharmacol . 2019;39(3):226 - 237; 6.) Relmada data on file Clinical data has demonstrated: • Robust, rapid, and sustained statistically significant antidepressant effects on all tested scales 4 • Rapid onset: significant efficacy effects by Day 4 4 • Favorable safety and tolerability profile consistent across all studies 4,5,6 : no opioid and no psychotomimetic adverse events and no metabolic side effects 4,5,6 • Orally administered, once - daily tablet Novel Mechanism of Action: preferential targeting of NMDAR channels potentially associated with MDD 1,2,3

©202 3 Relmada - All rights reserve d 8 REL - 1017 Phase 1 & 2 Efficacy and Safety Data
©202 3 Relmada - All rights reserve d All phase 1 studies for REL - 1017 are completed 9 Multiple Ascending Dose (MAD) study Single Ascending Dose (SAD) study Renal Impairment study and Hepatic Impairment study Drug - Drug Interaction (DDI) study Absorption, distribution, metabolism, excretion (ADME) study Oxycodone Human Abuse Potential (HAP) study Intravenous Ketamine Human Abuse Potential (HAP) study
©202 3 Relmada - All rights reserve d 10 “The d - isomer lacks significant respiratory depressant action and addiction liability...” US Drug Enforcement Administration December 2019 4 1.) Henningfield , et al. REL - 1017 ( esmethadone ; D - methadone) does not cause reinforcing effect, physical dependence and withdrawal signs in Sprague Dawley rats. Sci Rep 12, 1 1389 (2022); 2.) Shram M, et al., No meaningful abuse potential in recreational opioid users of REL - 1017 ( esmethadone hydrochloride), a new NMDAR antagonist and potential rapid - acting antidepressant American Society of Clinical Psychopharmacolog y (ASCP) 2022; 3.) Shram M, et al., No meaningful abuse potential in recreational ketamine users of REL - 1017 ( esmethadone hydrochloride), a new NMDAR antagonist and potential rapid - acting antidepressant. American Society of Clinical Psychopharmacolo gy (ASCP) 2022; 4.) US DEA Statement on Methadone, December 2019 February 2022 The results of experimental studies predictive of human abuse potential 1 and the results of human abuse potential studies in recreational opioid users 2 and in recreational ketamine users 3 indicate no meaningful abuse potential and support the DEA statement below:
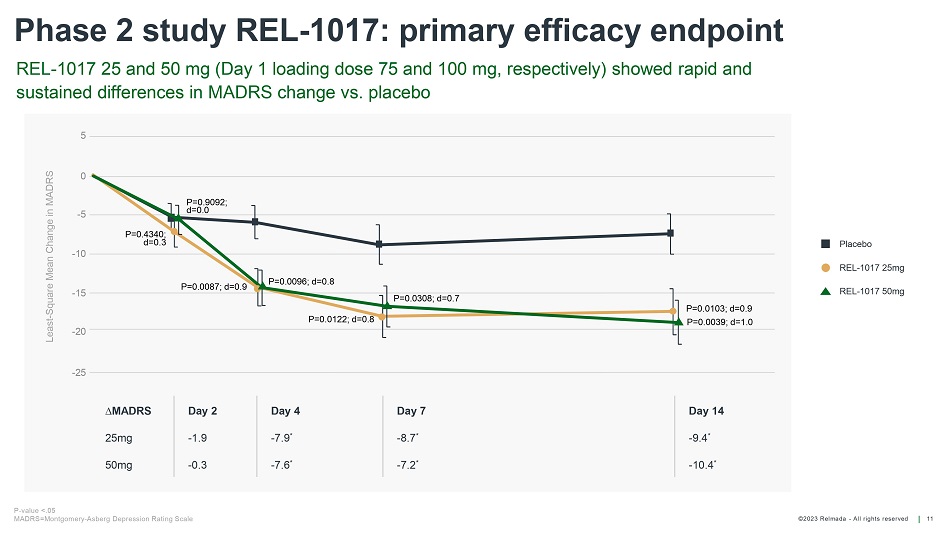
©202 3 Relmada - All rights reserve d Phase 2 study REL - 1017: primary efficacy endpoint 11 Least - Square Mean Change in MADRS 5 0 - 5 - 25 - 10 - 15 - 20 P=0.0103; d=0.9 P=0.0039; d=1.0 P=0.0308; d=0.7 P=0.0122; d=0.8 P=0.0096; d=0.8 P=0.0087; d=0.9 P=0.9092; d=0.0 P=0.4340; d=0.3 D MADRS Day 2 Day 4 Day 7 Day 14 25mg - 1.9 - 7.9 * - 8.7 * - 9.4 * 50mg - 0.3 - 7.6 * - 7.2 * - 10.4 * Placebo REL - 1017 25mg REL - 1017 50mg REL - 1017 25 and 50 mg (Day 1 loading dose 75 and 100 mg, respectively) showed rapid and sustained differences in MADRS change vs. placebo P - value <.05 MADRS=Montgomery - Asberg Depression Rating Scale
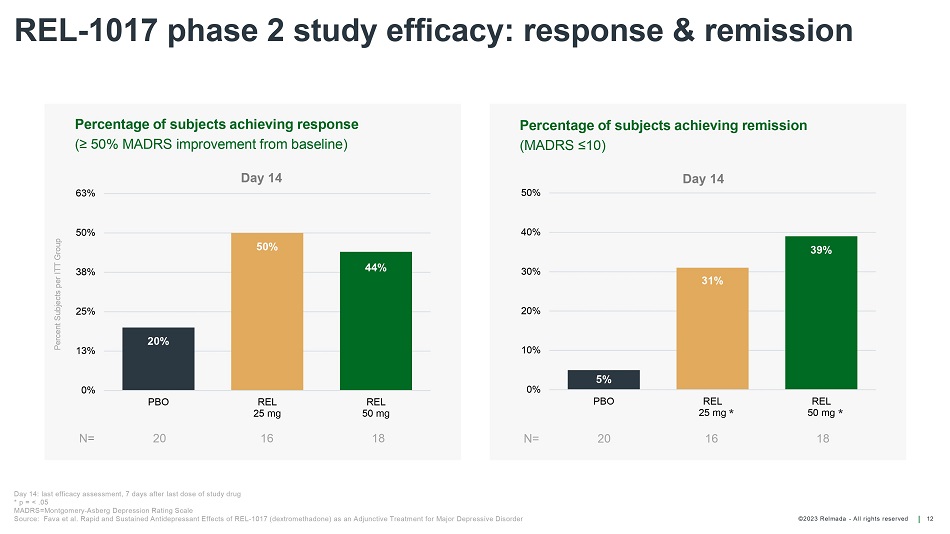
©202 3 Relmada - All rights reserve d REL - 1017 phase 2 study efficacy: response & remission 12 Percentage of s ubjects a chieving r emission (MADRS ≤10) 20% 50% 44% 0% 13% 25% 38% 50% 63% PBO REL 25 mg REL 50 mg Day 14 Percent Subjects per ITT Group Percentage of s ubjects a chieving r esponse (≥ 50% MADRS i mprovement from b aseline) N = 20 16 18 5% 31% 39% 0% 10% 20% 30% 40% 50% PBO REL 25 mg REL 50 mg Day 14 N= 20 16 18 Day 14: last efficacy assessment, 7 days after last dose of study drug * p = < .05 MADRS=Montgomery - Asberg Depression Rating Scale Source: Fava et al. Rapid and Sustained Antidepressant Effects of REL - 1017 ( dextromethadone ) as an Adjunctive Treatment for Major Depressive Disorder * *

©202 3 Relmada - All rights reserve d 13 Reliance: The Phase 3 Program for REL - 1017
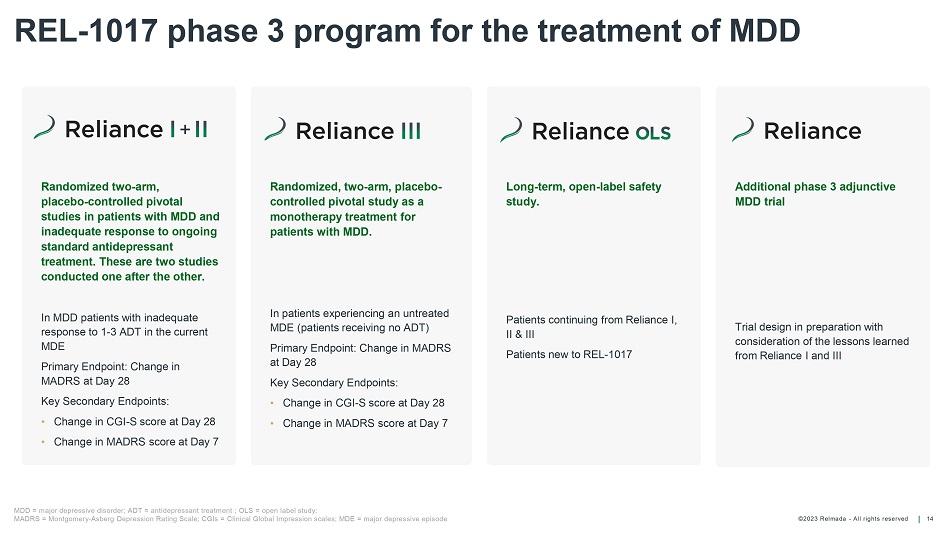
©202 3 Relmada - All rights reserve d Randomized, two - arm, placebo - controlled pivotal study as a monotherapy treatment for patients with MDD. In patients experiencing an untreated MDE (patients receiving no ADT) Primary Endpoint: Change in MADRS at Day 28 Key Secondary Endpoints: • Change in CGI - S score at Day 28 • Change in MADRS score at Day 7 Randomized two - arm, placebo - controlled pivotal studies in patients with MDD and inadequate response to ongoing standard antidepressant treatment. These are two studies conducted one after the other. In MDD patients with inadequate response to 1 - 3 ADT in the current MDE Primary Endpoint: Change in MADRS at Day 28 Key Secondary Endpoints: • Change in CGI - S score at Day 28 • Change in MADRS score at Day 7 Long - term, open - label safety study. Patients continuing from Reliance I, II & III Patients new to REL - 1017 REL - 1017 phase 3 program for the treatment of MDD 14 MDD = major depressive disorder; ADT = antidepressant treatment ; OLS = open label study; MADRS = Montgomery - Asberg Depression Rating Scale; CGIs = Clinical Global Impression scales; MDE = major depressive episode Additional phase 3 adjunctive MDD trial Trial design in preparation with consideration of the lessons learned from Reliance I and III +
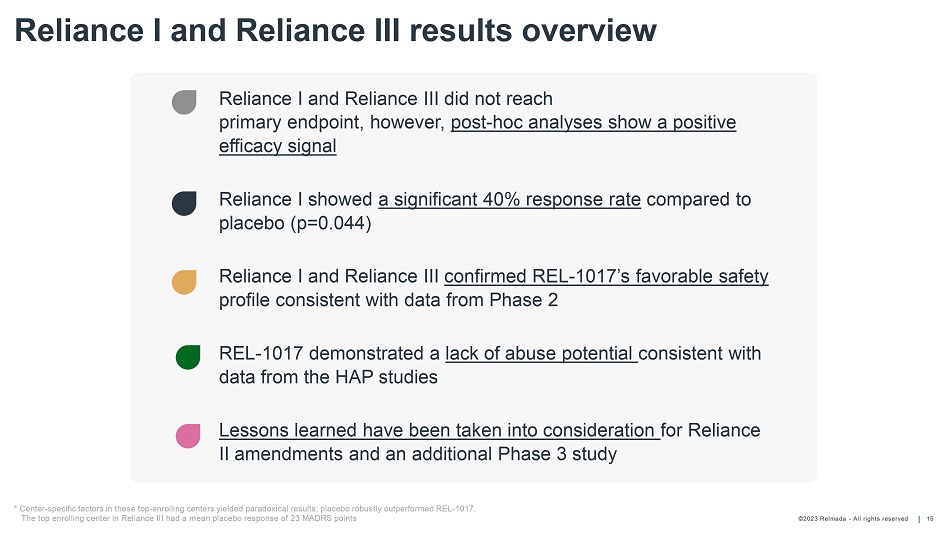
©202 3 Relmada - All rights reserve d Reliance I and Reliance III results overview 15 * Center - specific factors in these top - enrolling centers yielded paradoxical results: placebo robustly outperformed REL - 1017. The top enrolling center in Reliance III had a mean placebo response of 23 MADRS points Reliance I and Reliance III did not reach primary endpoint, however, post - hoc analyses show a positive efficacy signal Reliance I showed a significant 40% response rate compared to placebo (p=0.044) Reliance I and Reliance III confirmed REL - 1017’s favorable safety profile consistent with data fro m Phase 2 REL - 1017 demonstrated a lack of abuse potential consistent with data from the HAP studies Lessons learned have been taken into consideration for Reliance II amendments and an additional Phase 3 study
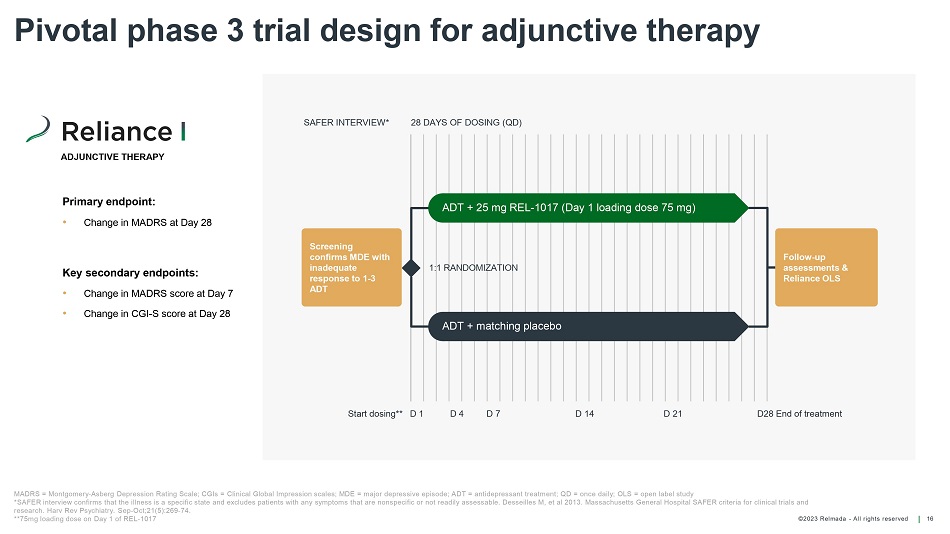
©202 3 Relmada - All rights reserve d Pivotal phase 3 trial design for adjunctive therapy 16 Primary endpoint: • Change in MADRS at Day 28 Key secondary endpoints: • Change in MADRS score at Day 7 • Change in CGI - S score at Day 28 Follow - up assessments & Reliance OLS 1:1 RANDOMIZATION 28 DAYS OF DOSING (QD) D28 End of t reatment ADT + 25 mg REL - 1017 (Day 1 loading dose 75 mg) ADT + matching placebo SAFER INTERVIEW* Screening confirms MDE with inadequate response to 1 - 3 ADT MADRS = Montgomery - Asberg Depression Rating Scale; CGIs = Clinical Global Impression scales; MDE = major depressive episode; ADT = antidepressant treat me nt; QD = once daily; OLS = open label study *SAFER interview confirms that the illness is a specific state and excludes patients with any symptoms that are nonspecific o r n ot readily assessable. Desseilles M, et al 2013. Massachusetts General Hospital SAFER criteria for clinical trials and research. Harv Rev Psychiatry. Sep - Oct;21(5):269 - 74. **75mg loading dose on Day 1 of REL - 1017 ADJUNCTIVE THERAPY Start dosing** D 1 D 4 D 7 D 14 D 21
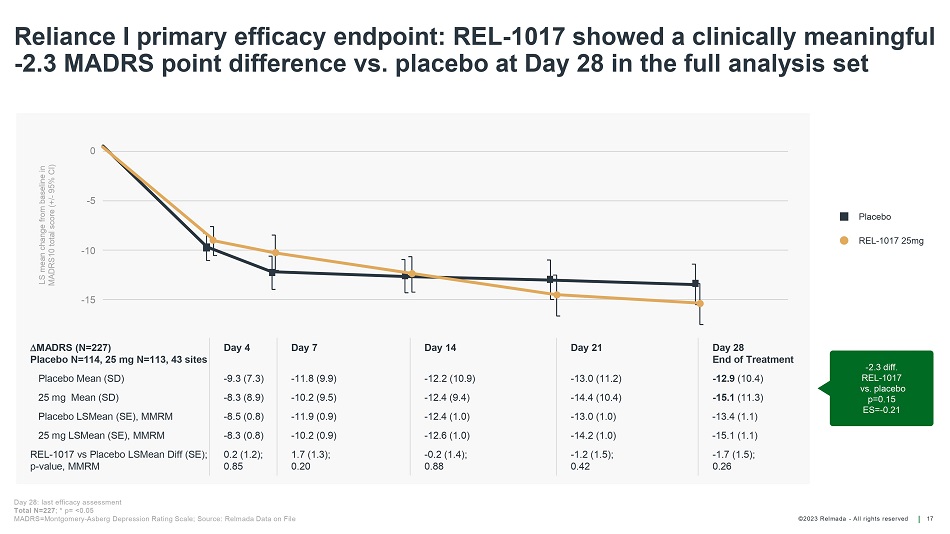
©202 3 Relmada - All rights reserve d LS mean change from baseline in MADRS10 total score (+/ - 95% CI) 0 - 5 - 10 - 15 Reliance I primary efficacy endpoint: REL - 1017 showed a clinically meaningful - 2.3 MADRS point difference vs. placebo at Day 28 in the full analysis set 17 Day 28: last efficacy assessment Total N=227 ; * p= <0.05 MADRS=Montgomery - Asberg Depression Rating Scale; Source: Relmada Data on File Placebo REL - 1017 25mg - 2.3 diff. REL - 1017 vs. placebo p=0.15 ES= - 0.21 MADRS (N=227) Placebo N=114, 25 mg N=113, 43 sites Day 4 Day 7 Day 14 Day 21 Day 28 End of Treatment Placebo Mean (SD) - 9.3 (7.3) - 11.8 (9.9) - 12.2 (10.9) - 13.0 (11.2) - 12.9 (10.4) 25 mg Mean (SD) - 8.3 (8.9) - 10.2 (9.5) - 12.4 (9.4) - 14.4 (10.4) - 15.1 (11.3) Placebo LSMean (SE), MMRM - 8.5 (0.8) - 11.9 (0.9) - 12.4 (1.0) - 13.0 (1.0) - 13.4 (1.1) 25 mg LSMean (SE), MMRM - 8.3 (0.8) - 10.2 (0.9) - 12.6 (1.0) - 14.2 (1.0) - 15.1 (1.1) REL - 1017 vs Placebo LSMean Diff (SE); p - value, MMRM 0.2 (1.2); 0.85 1.7 (1.3); 0.20 - 0.2 (1.4); 0.88 - 1.2 (1.5); 0.42 - 1.7 (1.5); 0.26
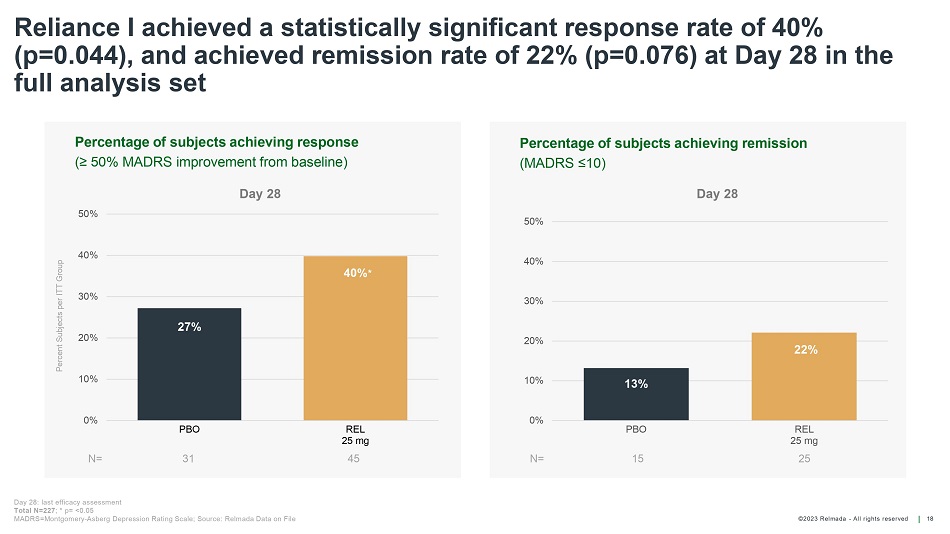
©202 3 Relmada - All rights reserve d Percentage of s ubjects a chieving r emission (MADRS ≤10) Percentage of s ubjects a chieving r esponse (≥ 50% MADRS i mprovement from b aseline) Reliance I achieved a statistically significant response rate of 40% (p=0.044), and achieved remission rate of 22% (p=0.076) at Day 28 in the full analysis set 18 N= 27% 40% 0% 10% 20% 30% 40% 50% PBO REL 25 mg 45 13% 22% 0% 10% 20% 30% 40% 50% PBO REL 25 mg 15 25 N= 31 * Day 28: last efficacy assessment Total N=227 ; * p= <0.05 MADRS=Montgomery - Asberg Depression Rating Scale; Source: Relmada Data on File Percent Subjects per ITT Group Day 28 Day 28
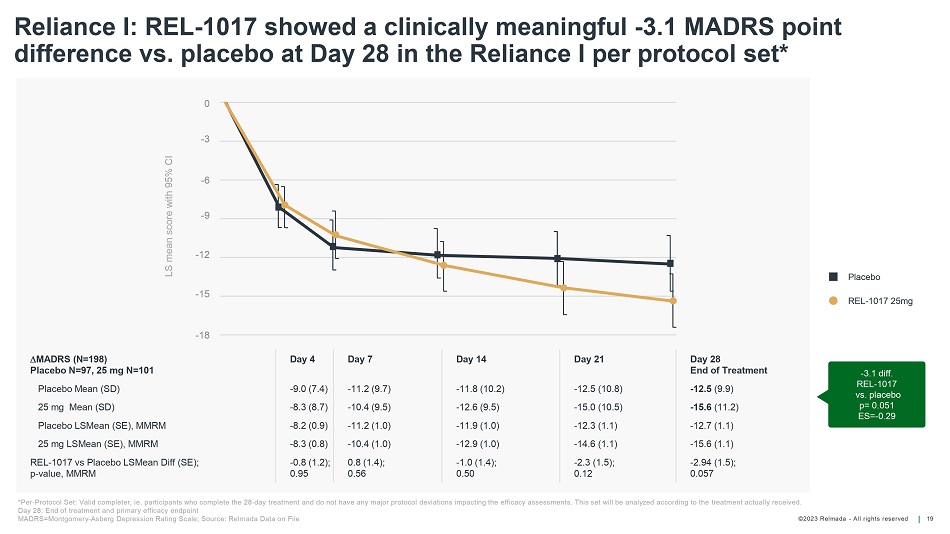
©202 3 Relmada - All rights reserve d Reliance I: REL - 1017 showed a clinically meaningful - 3.1 MADRS point difference vs. placebo at Day 28 in the Reliance I per protocol set* 19 - 3.1 diff. REL - 1017 vs. placebo p= 0.051 ES= - 0.29 LS mean score with 95% CI Placebo REL - 1017 25mg *Per - Protocol Set: Valid completer, ie , participants who complete the 28 - day treatment and do not have any major protocol deviations impacting the efficacy assessment s. This set will be analyzed according to the treatment actually received. Day 28: End of treatment and primary efficacy endpoint MADRS=Montgomery - Asberg Depression Rating Scale; Source: Relmada Data on File MADRS (N=198) Placebo N=97, 25 mg N=101 Day 4 Day 7 Day 14 Day 21 Day 28 End of Treatment Placebo Mean (SD) - 9.0 (7.4) - 11.2 (9.7) - 11.8 (10.2) - 12.5 (10.8) - 12.5 (9.9) 25 mg Mean (SD) - 8.3 (8.7) - 10.4 (9.5) - 12.6 (9.5) - 15.0 (10.5) - 15.6 (11.2) Placebo LSMean (SE), MMRM - 8.2 (0.9) - 11.2 (1.0) - 11.9 (1.0) - 12.3 (1.1) - 12.7 (1.1) 25 mg LSMean (SE), MMRM - 8.3 (0.8) - 10.4 (1.0) - 12.9 (1.0) - 14.6 (1.1) - 15.6 (1.1) REL - 1017 vs Placebo LSMean Diff (SE); p - value, MMRM - 0.8 (1.2); 0.95 0.8 (1.4); 0.56 - 1.0 (1.4); 0.50 - 2.3 (1.5); 0.12 - 2.94 (1.5); 0.057 0 - 3 - 15 - 6 - 9 - 12 - 1 8
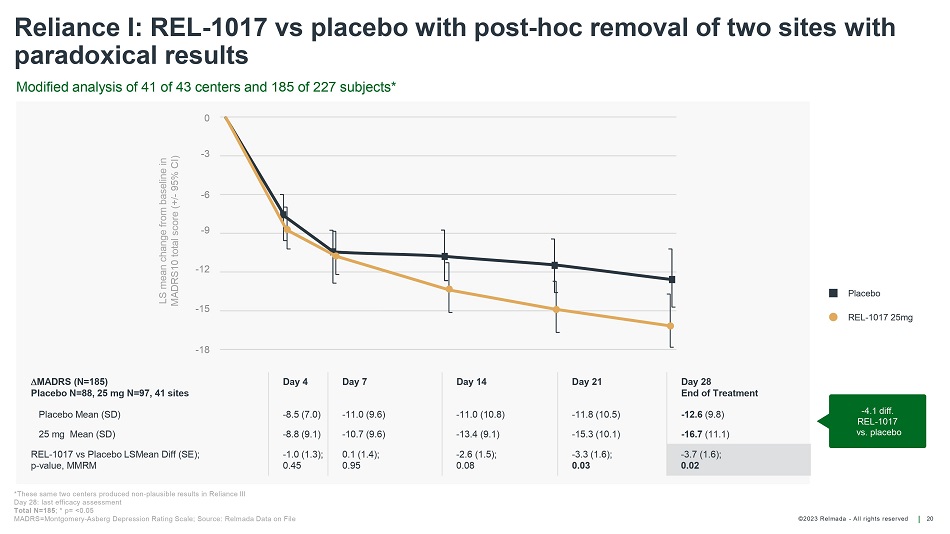
©202 3 Relmada - All rights reserve d 20 Reliance I: REL - 1017 vs placebo with post - hoc removal of two sites with paradoxical results - 4.1 diff. REL - 1017 vs. placebo MADRS (N=185) Placebo N=88, 25 mg N=97, 41 sites Day 4 Day 7 Day 14 Day 21 Day 28 End of Treatment Placebo Mean (SD) - 8.5 (7.0) - 11.0 (9.6) - 11.0 (10.8) - 11.8 (10.5) - 12.6 (9.8) 25 mg Mean (SD) - 8.8 (9.1) - 10.7 (9.6) - 13.4 (9.1) - 15.3 (10.1) - 16.7 (11.1) REL - 1017 vs Placebo LSMean Diff (SE); p - value, MMRM - 1.0 (1.3); 0.45 0.1 (1.4); 0.95 - 2.6 (1.5); 0.08 - 3.3 (1.6); 0.03 - 3.7 (1.6); 0.02 Modified analysis of 41 of 43 centers and 185 of 227 subjects* *These same two centers produced non - plausible results in Reliance III Day 28: last efficacy assessment Total N=185 ; * p= <0.05 MADRS=Montgomery - Asberg Depression Rating Scale; Source: Relmada Data on File Placebo REL - 1017 25mg LS mean change from baseline in MADRS10 total score (+/ - 95% CI) 0 - 3 - 15 - 6 - 9 - 12 - 1 8
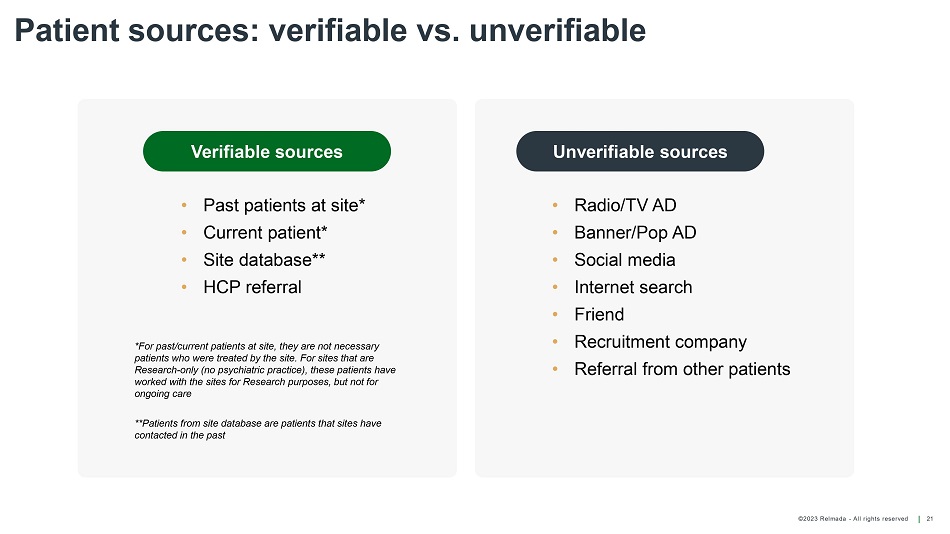
©202 3 Relmada - All rights reserve d Patient sources: verifiable vs. unverifiable 21 Verifiable sources • Past patients at site* • Current patient* • Site database** • HCP referral • Radio/TV AD • Banner/Pop AD • Social media • Internet search • Friend • Recruitment company • Referral from other patients *For past/current patients at site, they are not necessary patients who were treated by the site. For sites that are Research - only (no psychiatric practice), these patients have worked with the sites for Research purposes, but not for ongoing care **Patients from site database are patients that sites have contacted in the past Unverifiable sources
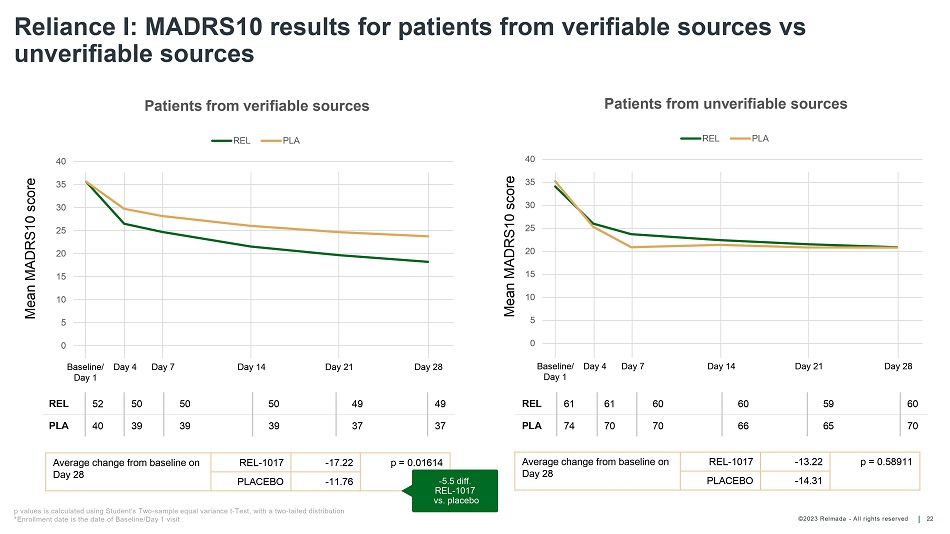
©202 3 Relmada - All rights reserve d Baseline/ Day 1 Day 4 Day 7 Day 14 Day 21 Day 28 Reliance I: MADRS10 results for patients from verifiable sources vs unverifiable sources 22 REL 52 50 50 50 49 49 PLA 40 39 39 39 37 37 Average change from baseline on Day 28 REL - 1017 - 17.22 p = 0.01614 PLACEBO - 11.76 Average change from baseline on Day 28 REL - 1017 - 13.22 p = 0.58911 PLACEBO - 14.31 Mean MADRS10 score Mean MADRS10 score Baseline/ Day 1 Day 4 Day 7 Day 14 Day 21 Day 28 REL 61 61 60 60 59 60 PLA 74 70 70 66 65 70 p values is calculated using Student's Two - sample equal variance t - Test, with a two - tailed distribution *Enrollment date is the date of Baseline/Day 1 visit 0 5 10 15 20 25 30 35 40 Patients from verifiable sources REL PLA - 5.5 diff. REL - 1017 vs. placebo 0 5 10 15 20 25 30 35 40 Patients from unverifiable sources REL PLA
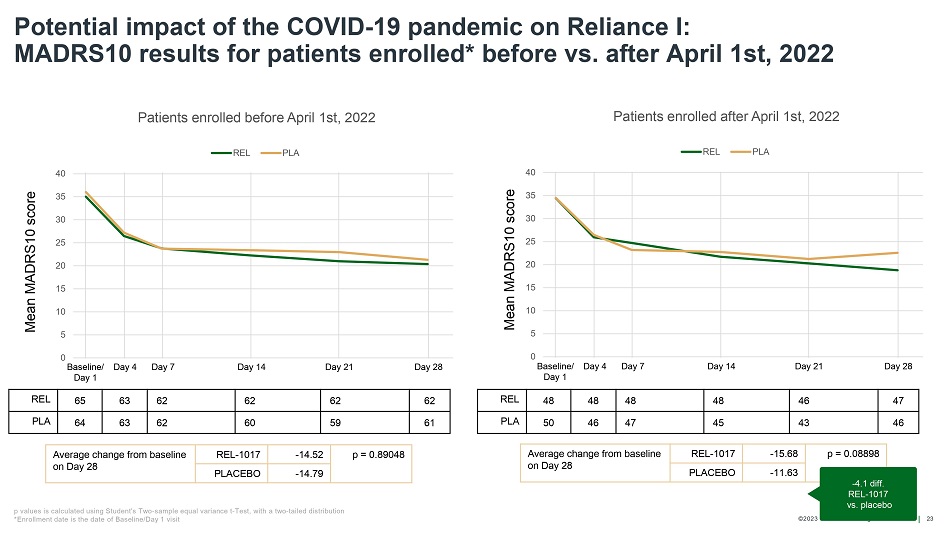
©202 3 Relmada - All rights reserve d Baseline/ Day 1 Day 4 Day 7 Day 14 Day 21 Day 28 Potential impact of the COVID - 19 pandemic on Reliance I: MADRS10 results for patients enrolled* before vs. after April 1st, 2022 23 REL 65 63 62 62 62 62 PLA 64 63 62 60 59 61 Average change from baseline on Day 28 REL - 1017 - 14.52 p = 0.89048 PLACEBO - 14.79 Average change from baseline on Day 28 REL - 1017 - 15.68 p = 0.08898 PLACEBO - 11.63 Mean MADRS10 score Mean MADRS10 score 0 5 10 15 20 25 30 35 40 Patients enrolled before April 1st, 2022 REL PLA Baseline/ Day 1 Day 4 Day 7 Day 14 Day 21 Day 28 0 5 10 15 20 25 30 35 40 Patients enrolled after April 1st, 2022 REL PLA REL 48 48 48 48 46 47 PLA 50 46 47 45 43 46 - 4.1 diff. REL - 1017 vs. placebo p values is calculated using Student's Two - sample equal variance t - Test, with a two - tailed distribution *Enrollment date is the date of Baseline/Day 1 visit
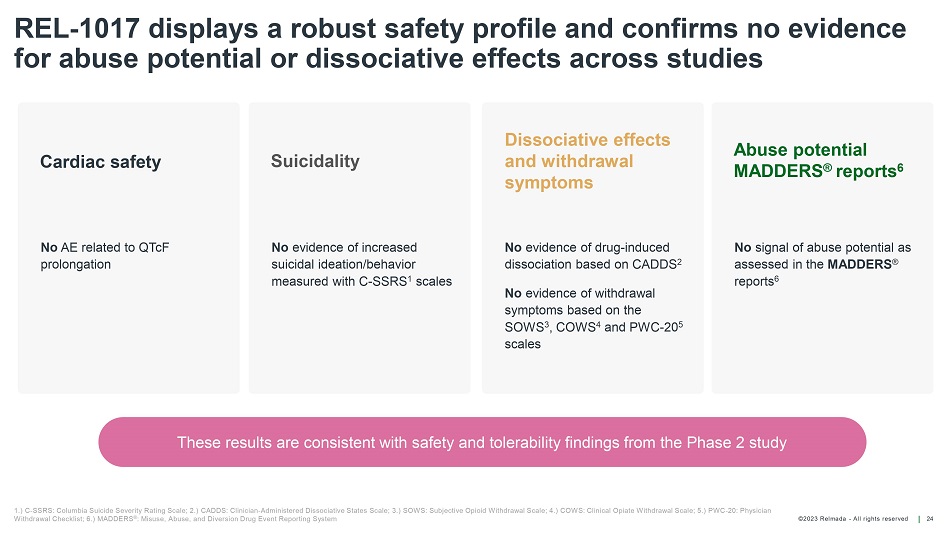
©202 3 Relmada - All rights reserve d REL - 1017 displays a robust safety profile and confirms no evidence for abuse potential or dissociative effects across studies 24 1.) C - SSRS: Columbia Suicide Severity Rating Scale; 2.) CADDS: Clinician - Administered Dissociative States Scale; 3.) SOWS: Subje ctive Opioid Withdrawal Scale; 4.) COWS: Clinical Opiate Withdrawal Scale; 5.) PWC - 20: Physician Withdrawal Checklist; 6.) MADDERS ® : Misuse, Abuse, and Diversion Drug Event Reporting System No AE related to QTcF prolongation No evidence of increased suicidal ideation/behavior measured with C - SSRS 1 scales No evidence of drug - induced dissociation based on CADDS 2 No evidence of withdrawal symptoms based on the SOWS 3 , COWS 4 and PWC - 20 5 scales No signal of abuse potential as assessed in the MADDERS ® reports 6 Cardiac safety Suicidality Dissociative effects and withdrawal symptoms Abuse potential MADDERS ® reports 6 These results are consistent with safety and tolerability findings from the Phase 2 study
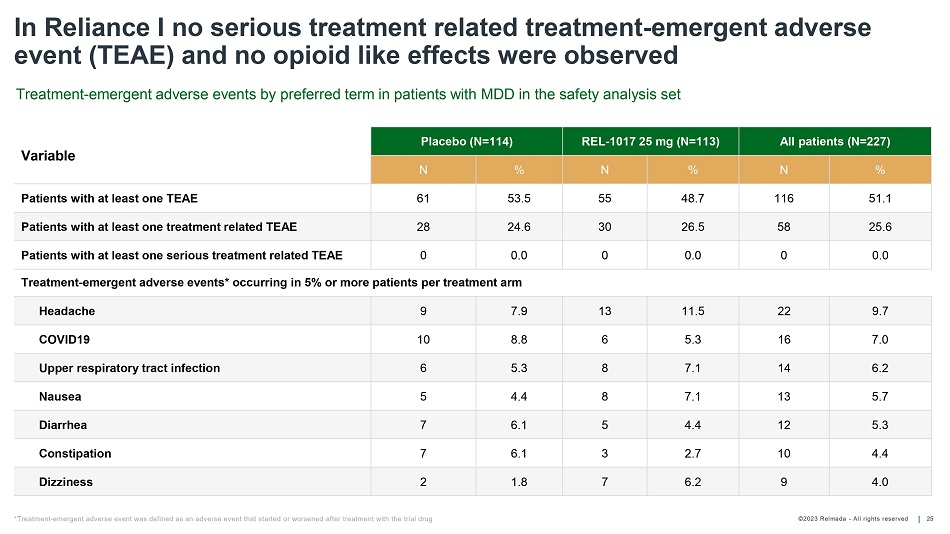
©202 3 Relmada - All rights reserve d In Reliance I no serious treatment related treatment - emergent adverse event (TEAE) and no opioid like effects were observed 25 Variable Placebo (N=114) REL - 1017 25 mg (N=113) All patients (N=227) N % N % N % Patients with at least one TEAE 61 53.5 55 48.7 116 51.1 Patients with at least one treatment related TEAE 28 24.6 30 26.5 58 25.6 Patients with at least one serious treatment related TEAE 0 0.0 0 0.0 0 0.0 Treatment - emergent adverse events* occurring in 5% or more patients per treatment arm Headache 9 7.9 13 11.5 22 9.7 COVID19 10 8.8 6 5.3 16 7.0 Upper respiratory tract infection 6 5.3 8 7.1 14 6.2 Nausea 5 4.4 8 7.1 13 5.7 Diarrhea 7 6.1 5 4.4 12 5.3 Constipation 7 6.1 3 2.7 10 4.4 Dizziness 2 1.8 7 6.2 9 4.0 Treatment - emergent adverse events by preferred term in patients with MDD in the safety analysis set *Treatment - emergent adverse event was defined as an adverse event that started or worsened after treatment with the trial drug

©202 3 Relmada - All rights reserve d Key learnings from stakeholders following Reliance I and Reliance III results Study site visits were too long and entailed too many assessments High enrolling sites with high placebo rates were over - represented in the final dataset Study screening eligibility adjudication needed improvement COVID - 19 impacted our trial due to the large number of patients experiencing situational depression related to isolation and other pandemic related issues 26 External stakeholders include KOLs, principal investigators, and study coordinators

©202 3 Relmada - All rights reserve d Changes to improve subject quality and better manage placebo response for ongoing and new studies 27 Requirement of medical records to verify depression diagnoses and ADT history to ensure enrollment of patients with true clinical depression Increased clinical trial oversight and management to improve screening eligibility adjudication Careful site selection based on the wealth of data gathered from recent trial experiences Limiting the number of patients enrolled per site to ensure there is not a disproportionate effect on study outcomes Protocol simplification to reduce the duration of site visits and assessments, enhance recruitment, and minimize placebo response
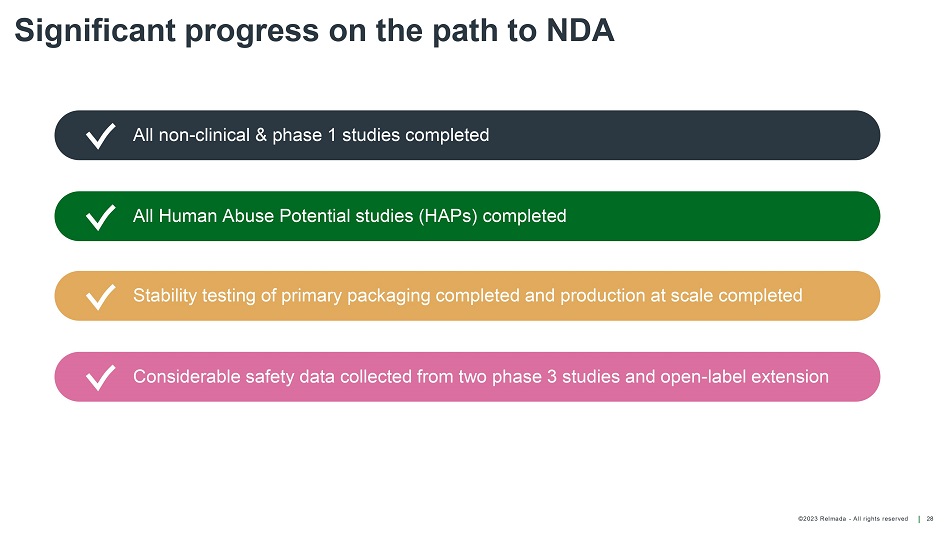
©202 3 Relmada - All rights reserve d Significant progress on the path to NDA 28 All non - clinical & phase 1 studies completed All Human Abuse Potential studies (HAPs) completed Stability testing of primary packaging completed and production at scale completed Considerable safety data collected from two phase 3 studies and open - label extension

©202 3 Relmada - All rights reserve d 29 Corporate Information
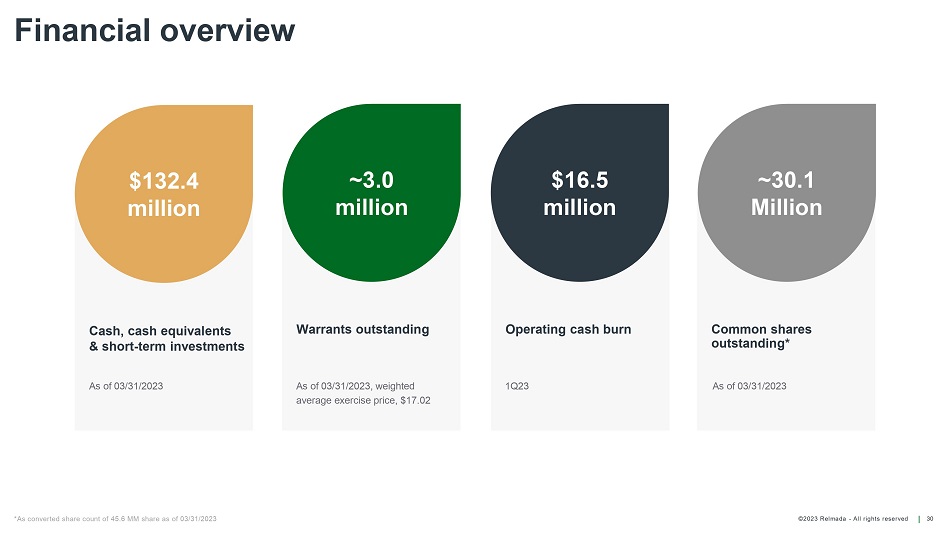
©202 3 Relmada - All rights reserve d Warrants outstanding Operating cash burn Common shares outstanding* Financial overview 30 Cash, cash equivalents & short - term investments $132.4 million ~3.0 million $16.5 million ~30.1 Million As of 03/31/2023, weighted average exercise price, $17.02 1 Q23 As of 03/31/2023 As of 03/31/2023 *As converted share count of 45.6 MM share as of 03/31/2023
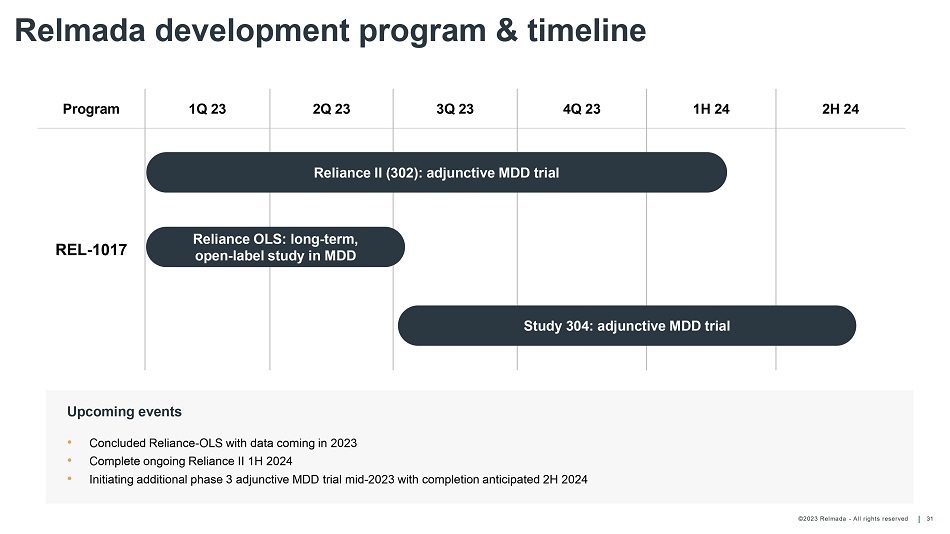
©202 3 Relmada - All rights reserve d Relmada development program & timeline 31 Program 1Q 23 2Q 23 3Q 23 4Q 23 1H 24 2H 24 REL - 1017 • Concluded Reliance - OLS with data coming in 2023 • Complete ongoing Reliance II 1H 2024 • Initiating additional phase 3 adjunctive MDD trial mid - 2023 with completion anticipated 2H 2024 Upcoming events Reliance II (302): adjunctive MDD trial Reliance OLS: long - term, open - label study in MDD Study 304: adjunctive MDD trial
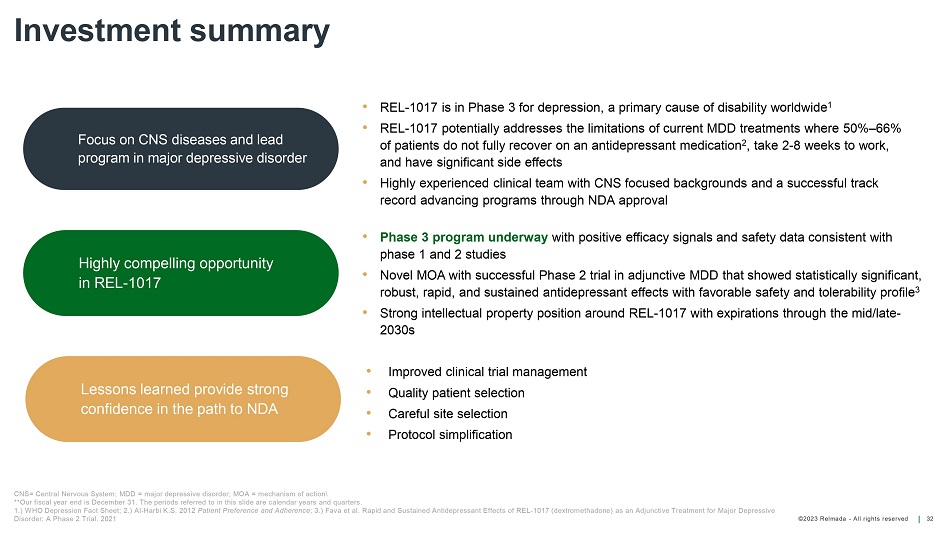
©202 3 Relmada - All rights reserve d Investment summary 32 Focus on CNS diseases and lead program in major depressive disorder CNS= Central Nervous System; MDD = major depressive disorder; MOA = mechanism of action \ **Our fiscal year end is December 31. The periods referred to in this slide are calendar years and quarters. 1.) WHO Depression Fact Sheet; 2.) Al - Harbi K.S. 2012 Patient Preference and Adherence ; 3.) Fava et al. Rapid and Sustained Antidepressant Effects of REL - 1017 ( dextromethadone ) as an Adjunctive Treatment for Major Depressive Disorder: A Phase 2 Trial. 2021 Highly compelling opportunity in REL - 1017 Lessons learned provide strong confidence in the path to NDA • Phase 3 program underway with positive efficacy signals and safety data consistent with phase 1 and 2 studies • Novel MOA with successful Phase 2 trial in adjunctive MDD that showed statistically significant, robust, rapid, and sustained antidepressant effects with favorable safety and tolerability profile 3 • Strong intellectual property position around REL - 1017 with expirations through the mid/late - 2030s • REL - 1017 is in Phase 3 for depression, a primary cause of disability worldwide 1 • REL - 1017 potentially addresses the limitations of current MDD treatments where 50% – 66% of patients do not fully recover on an antidepressant medication 2 , take 2 - 8 weeks to work, and have significant side effects • Highly experienced clinical team with CNS focused backgrounds and a successful track record advancing programs through NDA approval • Improved clinical trial management • Quality patient selection • Careful site selection • P rotocol simplification

Thank you 33